Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Vancomycin in Patients with External Ventricular Drain
- PMID: 37162349
- PMCID: PMC10269048
- DOI: 10.1128/aac.00241-23
Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Vancomycin in Patients with External Ventricular Drain
Abstract
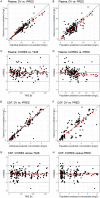
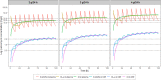
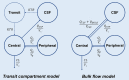
Vancomycin is a commonly used antibacterial agent in patients with primary central nervous system (CNS) infection. This study aims to examine predictors of vancomycin penetration into cerebrospinal fluid (CSF) in patients with external ventricular drainage and the feasibility of CSF sampling from the distal drainage port for therapeutic drug monitoring. Fourteen adult patients (9 with primary CNS infection) were treated with vancomycin intravenously. The vancomycin concentrations in blood and CSF (from proximal [CSF_P] and distal [CSF_D] drainage ports) were evaluated by population pharmacokinetics. Model-based simulations were conducted to compare various infusion modes. A three-compartment model with first-order elimination best described the vancomycin data. Estimated parameters included clearance (CL, 4.53 L/h), central compartment volume (Vc, 24.0 L), apparent CSF compartment volume (VCSF, 0.445 L), and clearance between central and CSF compartments (QCSF, 0.00322 L/h and 0.00135 L/h for patients with and without primary CNS infection, respectively). Creatinine clearance was a significant covariate on vancomycin CL. CSF protein was the primary covariate to explain the variability of QCSF. There was no detectable difference between the data for sampling from the proximal and the distal port. Intermittent infusion and continuous infusion with a loading dose reached the CSF target concentration faster than continuous infusion only. All infusion schedules reached similar CSF trough concentrations. Beyond adjusting doses according to renal function, starting treatment with a loading dose in patients with primary CSF infection is recommended. Occasionally, very high and possibly toxic doses would be required to achieve adequate CSF concentrations, which calls for more investigation of direct intraventricular administration of vancomycin. (This study has been registered at ClinicalTrials.gov under registration no. NCT04426383).
Keywords: CSF protein; central nervous system infection; distal port; population pharmacokinetics model; vancomycin; ventriculitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Blassmann U, Roehr AC, Frey OR, Vetter-Kerkhoff C, Thon N, Hope W, Briegel J, Huge V. 2016. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care 20:343. doi:10.1186/s13054-016-1523-y. - DOI - PMC - PubMed
-
- Rybak M, Lomaestro B, Rotschafer JC, Moellering R, Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi:10.2146/ajhp080434. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

