Mucins Shed from the Laminated Layer in Cystic Echinococcosis Are Captured by Kupffer Cells via the Lectin Receptor Clec4F
- PMID: 37162364
- PMCID: PMC10269144
- DOI: 10.1128/iai.00031-23
Mucins Shed from the Laminated Layer in Cystic Echinococcosis Are Captured by Kupffer Cells via the Lectin Receptor Clec4F
Erratum in
-
Correction for Barrios et al., "Mucins Shed from the Laminated Layer in Cystic Echinococcosis are Captured by Kupffer Cells via the Lectin Receptor Clec4F".Infect Immun. 2023 Nov 16;91(11):e0029923. doi: 10.1128/iai.00299-23. Epub 2023 Oct 17. Infect Immun. 2023. PMID: 37847030 Free PMC article. No abstract available.
Abstract
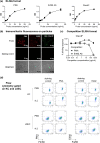
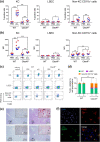
Cystic echinococcosis is caused by the larval stages (hydatids) of cestode parasites belonging to the species cluster Echinococcus granulosus sensu lato, with E. granulosus sensu stricto being the main infecting species. Hydatids are bladderlike structures that attain large sizes within various internal organs of livestock ungulates and humans. Hydatids are protected by the massive acellular laminated layer (LL), composed mainly of mucins. Parasite growth requires LL turnover, and abundant LL-derived particles are found at infection sites in infected humans, raising the question of how LL materials are dealt with by the hosts. In this article, we show that E. granulosus sensu stricto LL mucins injected into mice are taken up by Kupffer cells, the liver macrophages exposed to the vascular space. This uptake is largely dependent on the intact mucin glycans and on Clec4F, a C-type lectin receptor which, in rodents, is selectively expressed in Kupffer cells. This uptake mechanism operates on mucins injected both in soluble form intravenously (i.v.) and in particulate form intraperitoneally (i.p.). In mice harboring intraperitoneal infections by the same species, LL mucins were found essentially only at the infection site and in the liver, where they were taken up by Kupffer cells via Clec4F. Therefore, shed LL materials circulate in the host, and Kupffer cells can act as a sink for these materials, even when the parasite grows in sites other than the liver.
Keywords: Clec4F; Echinococcus; Kupffer cells; laminated layer; lectin; mucin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Cucher MA, Macchiaroli N, Baldi G, Camicia F, Prada L, Maldonado L, Avila HG, Fox A, Gutiérrez A, Negro P, López R, Jensen O, Rosenzvit M, Kamenetzky L. 2016. Cystic echinococcosis in South America: systematic review of species and genotypes of Echinococcus granulosus sensu lato in humans and natural domestic hosts. Trop Med Int Health 21:166–175. doi: 10.1111/tmi.12647. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

