This is a preprint.
Researching COVID to enhance recovery (RECOVER) pregnancy study: Rationale, objectives and design
- PMID: 37162923
- PMCID: PMC10168506
- DOI: 10.1101/2023.04.24.23289025
Researching COVID to enhance recovery (RECOVER) pregnancy study: Rationale, objectives and design
Abstract
Importance: Pregnancy induces unique physiologic changes to the immune response and hormonal changes leading to plausible differences in the risk of developing post-acute sequelae of SARS-CoV-2 (PASC), or Long COVID. Exposure to SARS-CoV-2 during pregnancy may also have long-term ramifications for exposed offspring, and it is critical to evaluate the health outcomes of exposed children. The National Institutes of Health (NIH) Researching COVID to Enhance Recovery (RECOVER) Multi-site Observational Study of PASC aims to evaluate the long-term sequelae of SARS-CoV-2 infection in various populations. RECOVER- Pregnancy was designed specifically to address long-term outcomes in maternal-child dyads.
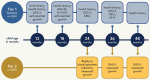
Methods: RECOVER-Pregnancy cohort is a combined prospective and retrospective cohort that proposes to enroll 2,300 individuals with a pregnancy during the COVID-19 pandemic and their offspring exposed and unexposed in utero, including single and multiple gestations. Enrollment will occur both in person at 27 sites through the Eunice Kennedy Shriver National Institutes of Health Maternal-Fetal Medicine Units Network and remotely through national recruitment by the study team at the University of California San Francisco (UCSF). Adults with and without SARS-CoV-2 infection during pregnancy are eligible for enrollment in the pregnancy cohort and will follow the protocol for RECOVER-Adult including validated screening tools, laboratory analyses and symptom questionnaires followed by more in-depth phenotyping of PASC on a subset of the overall cohort. Offspring exposed and unexposed in utero to SARS-CoV-2 maternal infection will undergo screening tests for neurodevelopment and other health outcomes at 12, 18, 24, 36 and 48 months of age. Blood specimens will be collected at 24 months of age for SARS-CoV-2 antibody testing, storage and anticipated later analyses proposed by RECOVER and other investigators.
Discussion: RECOVER-Pregnancy will address whether having SARS-CoV-2 during pregnancy modifies the risk factors, prevalence, and phenotype of PASC. The pregnancy cohort will also establish whether there are increased risks of adverse long-term outcomes among children exposed in utero.
Registration: NCT05172024.
Conflict of interest statement
Dr. Metz reports personal fees from Pfizer for her role as a medical consultant for a SARS-CoV-2 vaccination in pregnancy study, grants from Pfizer for role as a site PI for SARS-CoV-2 vaccination in pregnancy study, grants from Pfizer for role as a site PI for RSV vaccination in pregnancy study, and grants from Gestvision for role as a site PI for a preeclampsia study outside the submitted work. Dr. Horwitz reported serving as a member of the National Academy of Medicine Committee on the Long-Term Health Effects Stemming from COVID-19 and Implications for the Social Security Administration. Dr. Costantine reported receiving grant support for work not related to this paper from Baxter International and Siemens Healthcare and personal consulting fees not related to this paper from Progenity and Siemens Healthcare.
The other authors state that they have nothing to disclose.
Figures


References
-
- Bull-Otterson L. Post-COVID conditions among adult COVID-19 survivors aged 18-64 and ≥ 65 years old – United States, March 2020-November 2021. MMWR 2021; 71:713.
Publication types
Associated data
Grants and funding
- U10 HD040500/HD/NICHD NIH HHS/United States
- UG1 HD027869/HD/NICHD NIH HHS/United States
- UG1 HD087230/HD/NICHD NIH HHS/United States
- UM1 TR004409/TR/NCATS NIH HHS/United States
- OT2 HL161847/HL/NHLBI NIH HHS/United States
- UL1 TR002319/TR/NCATS NIH HHS/United States
- OT2 HL161841/HL/NHLBI NIH HHS/United States
- UG1 HD040500/HD/NICHD NIH HHS/United States
- OT2 HL156812/HL/NHLBI NIH HHS/United States
- UL1 TR002538/TR/NCATS NIH HHS/United States
- UG1 HD027915/HD/NICHD NIH HHS/United States
- UG1 HD087192/HD/NICHD NIH HHS/United States
- UG1 HD040544/HD/NICHD NIH HHS/United States
- UG1 HD034208/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
