This is a preprint.
Expansion of Disease Specific Cardiac Macrophages in Immune Checkpoint Inhibitor Myocarditis
- PMID: 37162929
- PMCID: PMC10168426
- DOI: 10.1101/2023.04.28.538426
Expansion of Disease Specific Cardiac Macrophages in Immune Checkpoint Inhibitor Myocarditis
Update in
-
Expansion of Pathogenic Cardiac Macrophages in Immune Checkpoint Inhibitor Myocarditis.Circulation. 2024 Jan 2;149(1):48-66. doi: 10.1161/CIRCULATIONAHA.122.062551. Epub 2023 Sep 25. Circulation. 2024. PMID: 37746718 Free PMC article.
Abstract
Background: Immune checkpoint inhibitors (ICIs), antibodies targeting PD-1/PD-L1 or CTLA4 have revolutionized cancer management but are associated with devastating immune-related adverse events (irAEs) including myocarditis. The main risk factor for ICI myocarditis is the use of combination PD-1 and CTLA4 inhibition. ICI-myocarditis is often fulminant and is pathologically characterized by myocardial infiltration of T lymphocytes and macrophages. While much has been learned regarding the role of T-cells in ICI-myocarditis, little is understood regarding the identity, transcriptional diversity, and functions of infiltrating macrophages.
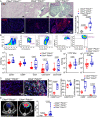
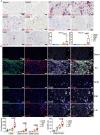
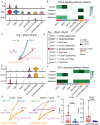
Methods: We employed an established murine ICI myocarditis model ( Ctla4 +/- Pdcd1 -/- mice) to explore the cardiac immune landscape using single-cell RNA-sequencing, immunostaining, flow cytometry, in situ RNA hybridization and molecular imaging and antibody neutralization studies.
Results: We observed marked increases in CCR2 + monocyte-derived macrophages and CD8 + T-cells in this model. The macrophage compartment was heterogeneous and displayed marked enrichment in an inflammatory CCR2 + subpopulation highly expressing Cxcl9 , Cxcl10 , Gbp2b , and Fcgr4 that originated from CCR2 + monocytes. Importantly, a similar macrophage population expressing CXCL9 , CXCL10 , and CD16α (human homologue of mouse FcgR4) was found selectively expanded in patients with ICI myocarditis compared to other forms of heart failure and myocarditis. In silico prediction of cell-cell communication suggested interactions between T-cells and Cxcl9 + Cxcl10 + macrophages via IFN-γ and CXCR3 signaling pathways. Depleting CD8 + T-cells, macrophages, and blockade of IFN-γ signaling blunted the expansion of Cxcl9 + Cxcl10 + macrophages in the heart and attenuated myocarditis suggesting that this interaction was necessary for disease pathogenesis.
Conclusion: These data demonstrate that ICI-myocarditis is associated with the expansion of a specific population of IFN-γ induced inflammatory macrophages and suggest the possibility that IFN-γ blockade may be considered as a treatment option for this devastating condition.
Figures





References
Publication types
Grants and funding
- R35 HL145212/HL/NHLBI NIH HHS/United States
- R01 HL151685/HL/NHLBI NIH HHS/United States
- R01 HL150891/HL/NHLBI NIH HHS/United States
- P30 AR073752/AR/NIAMS NIH HHS/United States
- P41 EB025815/EB/NIBIB NIH HHS/United States
- R01 HL156021/HL/NHLBI NIH HHS/United States
- R01 HL141466/HL/NHLBI NIH HHS/United States
- R01 HL131908/HL/NHLBI NIH HHS/United States
- R01 HL153436/HL/NHLBI NIH HHS/United States
- R01 HL151078/HL/NHLBI NIH HHS/United States
- R01 HL139714/HL/NHLBI NIH HHS/United States
- R01 HL138466/HL/NHLBI NIH HHS/United States
- R35 HL161185/HL/NHLBI NIH HHS/United States
- R01 HL160688/HL/NHLBI NIH HHS/United States
- S10 OD030403/OD/NIH HHS/United States
- R01 HL155990/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
