This is a preprint.
Senolytic therapy to modulate the progression of Alzheimer's Disease (SToMP-AD) - Outcomes from the first clinical trial of senolytic therapy for Alzheimer's disease
- PMID: 37162971
- PMCID: PMC10168460
- DOI: 10.21203/rs.3.rs-2809973/v1
Senolytic therapy to modulate the progression of Alzheimer's Disease (SToMP-AD) - Outcomes from the first clinical trial of senolytic therapy for Alzheimer's disease
Update in
-
Senolytic therapy in mild Alzheimer's disease: a phase 1 feasibility trial.Nat Med. 2023 Oct;29(10):2481-2488. doi: 10.1038/s41591-023-02543-w. Epub 2023 Sep 7. Nat Med. 2023. PMID: 37679434 Free PMC article. Clinical Trial.
Abstract
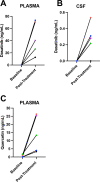
Cellular senescence has been identified as a pathological mechanism linked to tau and amyloid beta (Aβ) accumulation in mouse models of Alzheimer's disease (AD). Clearance of senescent cells using the senolytic compounds dasatinib (D) and quercetin (Q) reduced neuropathological burden and improved clinically relevant outcomes in the mice. Herein, we conducted a vanguard open-label clinical trial of senolytic therapy for AD with the primary aim of evaluating central nervous system (CNS) penetrance, as well as exploratory data collection relevant to safety, feasibility, and efficacy. Participants with early-stage symptomatic AD were enrolled in an open-label, 12-week pilot study of intermittent orally-delivered D+Q. CNS penetrance was assessed by evaluating drug levels in cerebrospinal fluid (CSF) using high performance liquid chromatography with tandem mass spectrometry. Safety was continuously monitored with adverse event reporting, vitals, and laboratory work. Cognition, neuroimaging, and plasma and CSF biomarkers were assessed at baseline and post-treatment. Five participants (mean age: 76±5 years; 40% female) completed the trial. The treatment increased D and Q levels in the blood of all participants ranging from 12.7 to 73.5 ng/ml for D and 3.29-26.30 ng/ml for Q. D levels were detected in the CSF of four participants ranging from 0.281 to 0.536 ng/ml (t(4)=3.123, p=0.035); Q was not detected. Treatment was well-tolerated with no early discontinuation and six mild to moderate adverse events occurring across the study. Cognitive and neuroimaging endpoints did not significantly differ from baseline to post-treatment. CNS levels of IL-6 and GFAP increased from baseline to post-treatment (t(4)=3.913, p=008 and t(4)=3.354, p=0.028, respectively) concomitant with decreased levels of several cytokines and chemokines associated with senescence, and a trend toward higher levels of Aβ42 (t(4)=-2.338, p=0.079). Collectively the data indicate the CNS penetrance of D and provide preliminary support for the safety, tolerability, and feasibility of the intervention and suggest that astrocytes and Aβ may be particularly responsive to the treatment. While early results are promising, fully powered, placebo-controlled studies are needed to evaluate the potential of AD modification with the novel approach of targeting cellular senescence.
Figures



References
-
- Prince M. J. et al. World Alzheimer Report 2015-The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. (2015).
-
- Aisen P. S. et al. The future of anti-amyloid trials. The Journal of Prevention of Alzheimer’s Disease 7, 146–151 (2020). - PubMed
-
- Korczyn A. D. Mixed dementia—the most common cause of dementia. Annals of the New York Academy of Sciences 977, 129–134 (2002). - PubMed
Publication types
Grants and funding
- T32 AG021890/AG/NIA NIH HHS/United States
- R01 AG065839/AG/NIA NIH HHS/United States
- R01 AG054076/AG/NIA NIH HHS/United States
- RF1 AG059421/AG/NIA NIH HHS/United States
- U01 AG046170/AG/NIA NIH HHS/United States
- R01 AG077472/AG/NIA NIH HHS/United States
- U24 AG059624/AG/NIA NIH HHS/United States
- TL1 TR002647/TR/NCATS NIH HHS/United States
- R01 AG068293/AG/NIA NIH HHS/United States
- P30 AG072947/AG/NIA NIH HHS/United States
- I01 BX005717/BX/BLRD VA/United States
- R33 AG061456/AG/NIA NIH HHS/United States
- R21 NS125171/NS/NINDS NIH HHS/United States
- P30 AG013319/AG/NIA NIH HHS/United States
- R01 AG068030/AG/NIA NIH HHS/United States
- U54 AG079754/AG/NIA NIH HHS/United States
- P30 AG066546/AG/NIA NIH HHS/United States
- R24 AG073199/AG/NIA NIH HHS/United States
- P30 AG044271/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

