This is a preprint.
A single-cell atlas of the aging murine ovary
- PMID: 37162983
- PMCID: PMC10168416
- DOI: 10.1101/2023.04.29.538828
A single-cell atlas of the aging murine ovary
Update in
-
A single-cell atlas of the aging mouse ovary.Nat Aging. 2024 Jan;4(1):145-162. doi: 10.1038/s43587-023-00552-5. Epub 2024 Jan 10. Nat Aging. 2024. PMID: 38200272 Free PMC article.
Abstract
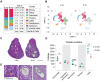
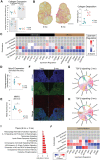
Ovarian aging leads to diminished fertility, dysregulated endocrine signaling, and increased chronic disease burden. These effects begin to emerge long before follicular exhaustion. Around 35 years old, women experience a sharp decline in fertility, corresponding to declines in oocyte quality. Despite a growing body of work, the field lacks a comprehensive cellular map of the transcriptomic changes in the aging ovary to identify early drivers of ovarian decline. To fill this gap, we performed single-cell RNA sequencing on ovarian tissue from young (3-month-old) and reproductively aged (9-month-old) mice. Our analysis revealed a doubling of immune cells in the aged ovary, with lymphocyte proportions increasing the most, which was confirmed by flow cytometry. We also found an age-related downregulation of collagenase pathways in stromal fibroblasts, which corresponds to rises in ovarian fibrosis. Follicular cells displayed stress response, immunogenic, and fibrotic signaling pathway inductions with aging. This report raises provides critical insights into mechanisms responsible for ovarian aging phenotypes.
Keywords: collagen; fibrosis; menopause; mouse; multinucleated giant cells; reproduction.
Conflict of interest statement
COMPLETING INTERESTS The authors declare no conflicts or competing interests.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
