This is a preprint.
The Phenotypic Spectrum of COL4A3 Heterozygotes
- PMID: 37163122
- PMCID: PMC10168410
- DOI: 10.1101/2023.04.11.23288298
The Phenotypic Spectrum of COL4A3 Heterozygotes
Update in
-
The Phenotypic Spectrum of COL4A3 Heterozygotes.Kidney Int Rep. 2023 Jul 25;8(10):2088-2099. doi: 10.1016/j.ekir.2023.07.010. eCollection 2023 Oct. Kidney Int Rep. 2023. PMID: 37849993 Free PMC article.
Abstract
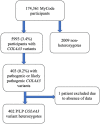
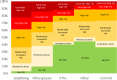
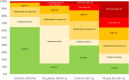
Most data on Alport Syndrome (AS) due to COL4A3 are limited to families with autosomal recessive AS or severe manifestations such as focal segmental glomerulosclerosis (FSGS). Using data from 174,418 participants in the Geisinger MyCode/DiscovEHR study, an unselected health system-based cohort with whole exome sequencing, we identified 403 participants (0.2%) who were heterozygous for likely pathogenic COL4A3 variants. Phenotypic data was evaluated using International Classification of Diseases (ICD) codes, laboratory data, and chart review. To evaluate the phenotypic spectrum of genetically-determined autosomal dominant AS, we matched COL4A3 heterozygotes 1:5 to non-heterozygotes using propensity scores by demographics, hypertension, diabetes, and nephrolithiasis. COL4A3 heterozygotes were at significantly increased risks of hematuria, decreased estimated glomerular filtration rate (eGFR), albuminuria, and end-stage kidney disease (ESKD) (p<0.05 for all comparisons) but not bilateral sensorineural hearing loss (p=0.9). Phenotypic severity tended to be more severe among patients with glycine missense variants located within the collagenous domain. For example, patients with Gly695Arg (n=161) had markedly increased risk of dipstick hematuria (OR 9.47, 95% CI: 6.30, 14.22) and ESKD diagnosis (OR 7.01, 95% CI: 3.48, 14.12) whereas those with PTVs (n=119) had moderately increased risks of dipstick hematuria (OR 1.63, 95% CI: 1.03, 2.58) and ESKD diagnosis (OR 3.43, 95% CI: 1.28, 9.19). Less than a third of patients had albuminuria screening completed, and fewer than 1/3 were taking inhibitors of the renin-angiotensin-aldosterone system (RAASi). Future studies are needed to evaluate the impact of earlier diagnosis, appropriate evaluation, and treatment of ADAS.
Conflict of interest statement
Conflicts of interest: No potential conflicts of interest relevant to this article were reported.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
