This is a preprint.
Durability of immunity and clinical protection in nursing home residents following bivalent SARS-CoV-2 vaccination
- PMID: 37163130
- PMCID: PMC10168517
- DOI: 10.1101/2023.04.25.23289050
Durability of immunity and clinical protection in nursing home residents following bivalent SARS-CoV-2 vaccination
Update in
-
Durability of immunity and clinical protection in nursing home residents following bivalent SARS-CoV-2 vaccination.EBioMedicine. 2024 Jul;105:105180. doi: 10.1016/j.ebiom.2024.105180. Epub 2024 Jun 10. EBioMedicine. 2024. PMID: 38861869 Free PMC article.
Abstract
Background: Vaccines have substantially mitigated the disproportional impact of SARS-CoV-2 on the high morbidity and mortality experienced by nursing home residents. However, variation in vaccine efficacy, immune senescence and waning immunity all undermine vaccine effectiveness over time. The introduction of the bivalent vaccine in September 2022 aimed to counter this increasing susceptibility and consequences of breakthrough infection, however data on the durability and protection of the vaccine are limited. We evaluated the durability of immunity and protection after the first bivalent vaccination to SARS-CoV-2 in nursing home residents.
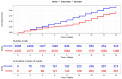
Methods: For the immunologic evaluation, community nursing home volunteers agreed to serial blood sampling before, at two weeks, three and six months after each vaccination for antibodies to spike protein and pseudovirus neutralization activity over time. Concurrent clinical outcomes were evaluated by reviewing electronic health record data from residents living in Veterans Administration managed nursing home units. Residents without recent infection but prior vaccination to SARS-CoV-2 were followed over time beginning with administration of the newly available bivalent vaccine using a target trial emulation (TTE) approach; TTE compared time to breakthrough infection, hospitalization and death between those who did and did not receive the bivalent vaccine.
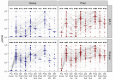
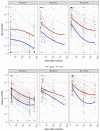
Results: We evaluated antibodies in 650 nursing home residents; 452 had data available following a first monovalent booster, 257 following a second monovalent booster and 321 following a bivalent vaccine. We found a rise in BA.5 neutralization activity from the first and second monovalent boosters through the bivalent vaccination regardless of prior SARS-CoV-2 history. Titers declined at three and six months after the bivalent vaccination but generally exceeded those at three months compared to either prior boost. BA.5 neutralization titers six months after the bivalent vaccination were diminished but had detectable levels in 80% of infection-naive and 100% of prior infected individuals. TTE evaluated 5903 unique subjects, of whom 2235 received the bivalent boost. TTE demonstrated 39% or greater reduction in risk of infection, hospitalization or death at four months following the bivalent boost.
Conclusion: Immunologic results mirrored those of the TTE and suggest bivalent vaccination added substantial protection for up to six months after bivalent vaccination with notable exceptions. However, the level of protection declined over this period, and by six months may open a window of added vulnerability to infection before the next updated vaccine becomes available. We strongly agree with the CDC recommendation that those who have not received a bivalent vaccination receive that now and these results support a second bivalent booster for those at greatest risk which includes many nursing home residents.
Figures


References
-
- CDC. Apr. 7, 2023. 2023. Risk for COVID-19 Infection, Hospitalization, and Death By Age Group. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-disc....
-
- Canaday DH, Oyebanji OA, Keresztesy D, Payne M, Wilk D, Carias L, Aung H, St Denis K, Lam EC, Rowley CF, Berry SD, Cameron CM, Cameron MJ, Wilson BM, Balazs AB, King CL, Gravenstein S. 2021. Significant Reduction in Vaccine-Induced Antibody Levels and Neutralization Activity Among Healthcare Workers and Nursing Home Residents 6 Months Following Coronavirus Disease 2019 BNT162b2 mRNA Vaccination. Clin Infect Dis doi:10.1093/cid/ciab963. - DOI - PMC - PubMed
-
- Nugent C, Abul Y, White EM, Shehadeh F, Kaczynski M, Felix LO, Ganesan N, Oyebanji OA, Vishnepolskiy I, Didion EM, Paxitzis A, Sheehan ML, Chan PA, Pfeifer WM, Dickerson E, Wilson BM, Mylonakis E, Kamaojjala S, King CL, Balazs AB, Canaday DH, Gravenstein S. 2023. Second monovalent SARS-CoV-2 mRNA booster restores Omicron-specific neutralizing activity in both nursing home residents and health care workers. Vaccine doi:10.1016/j.vaccine.2023.04.034. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
