Effects of Specialist Palliative Care for Patients Undergoing Major Abdominal Surgery for Cancer: A Randomized Clinical Trial
- PMID: 37163249
- PMCID: PMC10173099
- DOI: 10.1001/jamasurg.2023.1396
Effects of Specialist Palliative Care for Patients Undergoing Major Abdominal Surgery for Cancer: A Randomized Clinical Trial
Abstract
Importance: Specialist palliative care benefits patients undergoing medical treatment of cancer; however, data are lacking on whether patients undergoing surgery for cancer similarly benefit from specialist palliative care.
Objective: To determine the effect of a specialist palliative care intervention on patients undergoing surgery for cure or durable control of cancer.
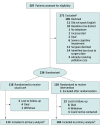
Design, setting, and participants: This was a single-center randomized clinical trial conducted from March 1, 2018, to October 28, 2021. Patients scheduled for specified intra-abdominal cancer operations were recruited from an academic urban referral center in the Southeastern US.
Intervention: Preoperative consultation with palliative care specialists and postoperative inpatient and outpatient palliative care follow-up for 90 days.
Main outcomes and measures: The prespecified primary end point was physical and functional quality of life (QoL) at postoperative day (POD) 90, measured by the Functional Assessment of Cancer Therapy-General (FACT-G) Trial Outcome Index (TOI), which is scored on a range of 0 to 56 with higher scores representing higher physical and functional QoL. Prespecified secondary end points included overall QoL at POD 90 measured by FACT-G, days alive at home until POD 90, and 1-year overall survival. Multivariable proportional odds logistic regression and Cox proportional hazards regression models were used to test the hypothesis that the intervention improved each of these end points relative to usual care in an intention-to-treat analysis.
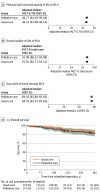
Results: A total of 235 eligible patients (median [IQR] age, 65.0 [56.8-71.1] years; 141 male [60.0%]) were randomly assigned to the intervention or usual care group in a 1:1 ratio. Specialist palliative care was received by 114 patients (97%) in the intervention group and 1 patient (1%) in the usual care group. Adjusted median scores on the FACT-G TOI measure of physical and functional QoL did not differ between groups (intervention score, 46.77; 95% CI, 44.18-49.04; usual care score, 46.23; 95% CI, 43.08-48.14; P = .46). Intervention vs usual care group odds ratio (OR) was 1.17 (95% CI, 0.77-1.80). Palliative care did not improve overall QoL measured by the FACT-G score (intervention vs usual care OR, 1.09; 95% CI, 0.75-1.58), days alive at home (OR, 0.87; 95% CI, 0.69-1.11), or 1-year overall survival (hazard ratio, 0.97; 95% CI, 0.50-1.88).
Conclusions and relevance: This randomized clinical trial showed no evidence that early specialist palliative care improves the QoL of patients undergoing nonpalliative cancer operations.
Trial registration: ClinicalTrials.gov Identifier: NCT03436290.
Conflict of interest statement
Figures
Comment in
-
Surgical Palliative Care-Who, When, and Why?JAMA Surg. 2023 Jul 1;158(7):755. doi: 10.1001/jamasurg.2023.1406. JAMA Surg. 2023. PMID: 37163247 No abstract available.
-
Palliative Care for Patients Undergoing Surgery.JAMA Surg. 2024 Feb 1;159(2):229. doi: 10.1001/jamasurg.2023.4514. JAMA Surg. 2024. PMID: 37755823 No abstract available.

