Diet-mediated constitutive induction of novel IL-4+ ILC2 cells maintains intestinal homeostasis in mice
- PMID: 37163450
- PMCID: PMC10174189
- DOI: 10.1084/jem.20221773
Diet-mediated constitutive induction of novel IL-4+ ILC2 cells maintains intestinal homeostasis in mice
Abstract
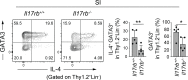
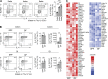
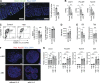
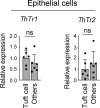
Group 2 innate lymphoid cells (ILC2s) expressing IL-5 and IL-13 are localized at various mucosal tissues and play critical roles in the induction of type 2 inflammation, response to helminth infection, and tissue repair. Here, we reveal a unique ILC2 subset in the mouse intestine that constitutively expresses IL-4 together with GATA3, ST2, KLRG1, IL-17RB, and IL-5. In this subset, IL-4 expression is regulated by mechanisms similar to but distinct from those observed in T cells and is partly affected by IL-25 signaling. Although the absence of the microbiota had marginal effects, feeding mice with a vitamin B1-deficient diet compromised the number of intestinal IL-4+ ILC2s. The decrease in the number of IL-4+ ILC2s caused by the vitamin B1 deficiency was accompanied by a reduction in IL-25-producing tuft cells. Our findings reveal that dietary vitamin B1 plays a critical role in maintaining interaction between tuft cells and IL-4+ ILC2s, a previously uncharacterized immune cell population that may contribute to maintaining intestinal homeostasis.
© 2023 Cui et al.
Conflict of interest statement
Disclosures: Y. Nagano reported personal fees from Astellas Pharma outside the submitted work. K. Honda reported being a scientific advisory board member of Vedanta Biosciences and 4BIO CAPITAL. No other disclosures were reported.
Figures








References
-
- Campbell, L., Hepworth M.R., Whittingham-Dowd J., Thompson S., Bancroft A.J., Hayes K.S., Shaw T.N., Dickey B.F., Flamar A.L., Artis D., et al. . 2019. ILC2s mediate systemic innate protection by priming mucus production at distal mucosal sites. J. Exp. Med. 216:2714–2723. 10.1084/jem.20180610 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

