A validated protocol to UV-inactivate SARS-CoV-2 and herpesvirus-infected cells
- PMID: 37163509
- PMCID: PMC10171616
- DOI: 10.1371/journal.pone.0274065
A validated protocol to UV-inactivate SARS-CoV-2 and herpesvirus-infected cells
Abstract
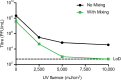
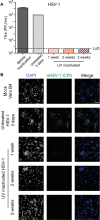
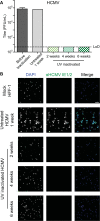
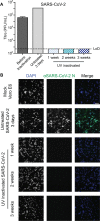
Downstream analysis of virus-infected cell samples, such as reverse transcription polymerase chain reaction (RT PCR) or mass spectrometry, often needs to be performed at lower biosafety levels than their actual cultivation, and thus the samples require inactivation before they can be transferred. Common inactivation methods involve chemical crosslinking with formaldehyde or denaturing samples with strong detergents, such as sodium dodecyl sulfate. However, these protocols destroy the protein quaternary structure and prevent the analysis of protein complexes, albeit through different chemical mechanisms. This often leads to studies being performed in over-expression or surrogate model systems. To address this problem, we generated a protocol that achieves the inactivation of infected cells through ultraviolet (UV) irradiation. UV irradiation damages viral genomes and crosslinks nucleic acids to proteins but leaves the overall structure of protein complexes mostly intact. Protein analysis can then be performed from intact cells without biosafety containment. While UV treatment protocols have been established to inactivate viral solutions, a protocol was missing to inactivate crude infected cell lysates, which heavily absorb light. In this work, we develop and validate a UV inactivation protocol for SARS-CoV-2, HSV-1, and HCMV-infected cells. A fluence of 10,000 mJ/cm2 with intermittent mixing was sufficient to completely inactivate infected cells, as demonstrated by the absence of viral replication even after three sequential passages of cells inoculated with the treated material. The herein described protocol should serve as a reference for inactivating cells infected with these or similar viruses and allow for the analysis of protein quaternary structure from bona fide infected cells.
Copyright: © 2023 Soh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Nishisaka-Nonaka R, Mawatari K, Yamamoto T, Kojima M, Shimohata T, Uebanso T, et al.. Irradiation by ultraviolet light-emitting diodes inactivates influenza a viruses by inhibiting replication and transcription of viral RNA in host cells. J Photochem Photobiol B. 2018;189:193–200. doi: 10.1016/j.jphotobiol.2018.10.017 . - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

