Creation of a Single Institutional Review Board for Collaborative Research in Nephrology: The APOLLO Experience
- PMID: 37163584
- PMCID: PMC10578633
- DOI: 10.2215/CJN.0000000000000197
Creation of a Single Institutional Review Board for Collaborative Research in Nephrology: The APOLLO Experience
Conflict of interest statement
A.A. Alexander reports an advisory or leadership role for Boston Children's Hospital. B.I. Freedman reports employment with Wake Forest University School of Medicine and Health Systems Management, Inc.; consultancy for AstraZeneca Pharmaceuticals, RenalytixAI, and Xinthera; research funding from AstraZeneca Pharmaceuticals and RenalytixAI; advisory or leadership roles on Editorial Boards of
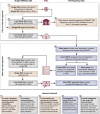
Figures
References
-
- Code of Federal Regulations. 45 CFR.46.114 Cooperative Research. Accessed November 13, 2022. https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46/su...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

