Human Papillomavirus Vaccination
- PMID: 37163625
- PMCID: PMC11567082
- DOI: 10.1056/NEJMcp2108502
Human Papillomavirus Vaccination
Abstract
A 24-year-old woman is being seen for routine health care. She has not received any vaccinations against human papillomavirus (HPV). The patient initiated sexual activity at 18 years of age and has had three male sex partners. What would you recommend regarding HPV vaccination?
Figures
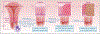

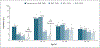
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
