Trajectory and Demographic Correlates of Antibodies to SARS-CoV-2 Nucleocapsid in Recently Infected Blood Donors, United States
- PMID: 37163762
- PMCID: PMC10310359
- DOI: 10.3201/eid2907.230173
Trajectory and Demographic Correlates of Antibodies to SARS-CoV-2 Nucleocapsid in Recently Infected Blood Donors, United States
Abstract
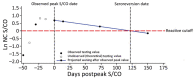
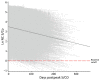
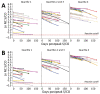
We evaluated antibodies to the nucleocapsid protein of SARS-CoV-2 in a large cohort of blood donors in the United States who were recently infected with the virus. Antibodies to the nucleocapsid protein of SARS-CoV-2 indicate previous infection but are subject to waning, potentially affecting epidemiologic studies. We longitudinally evaluated a cohort of 19,323 blood donors who had evidence of recent infection by using a widely available serologic test to determine the dynamics of such waning. We analyzed overall signal-to-cutoff values for 48,330 donations (average 2.5 donations/person) that had an average observation period of 102 days. The observed peak signal-to-cutoff value varied widely, but the waning rate was consistent across the range, with a half-life of 122 days. Within the cohort, only 0.75% of persons became seronegative. Factors predictive of higher peak values and longer time to seroreversion included increasing age, male sex, higher body mass index, and non-Caucasian race.
Keywords: COVID-19; N antibody; S antibody; SARS-CoV-2; United States; antibody; blood donors; coronavirus disease; coronaviruses; demographic correlates; half-life; nucleocapsid; respiratory infections; severe acute respiratory syndrome coronavirus 2; spike protein; trajectory; viruses; waning; zoonoses.
Figures

References
-
- Steele WR, Dodd RY, Notari EP, Xu M, Nelson D, Kessler DA, et al.; Transfusion-Transmissible Infections Monitoring System (TTIMS). Prevalence of human immunodeficiency virus, hepatitis B virus, and hepatitis C virus in United States blood donations, 2015 to 2019: The Transfusion-Transmissible Infections Monitoring System (TTIMS). Transfusion. 2020;60:2327–39. 10.1111/trf.16005 - DOI - PubMed
-
- Van Elslande J, Oyaert M, Lorent N, Vande Weygaerde Y, Van Pottelbergh G, Godderis L, et al. Lower persistence of anti-nucleocapsid compared to anti-spike antibodies up to one year after SARS-CoV-2 infection. Diagn Microbiol Infect Dis. 2022;103:115659. 10.1016/j.diagmicrobio.2022.115659 - DOI - PMC - PubMed
-
- Krutikov M, Palmer T, Tut G, Fuller C, Azmi B, Giddings R, et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): prospective cohort study in England. Lancet Healthy Longev. 2022;3:e13–21. 10.1016/S2666-7568(21)00282-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

