Optimization of energy production and central carbon metabolism in a non-respiring eukaryote
- PMID: 37164017
- PMCID: PMC7615655
- DOI: 10.1016/j.cub.2023.04.046
Optimization of energy production and central carbon metabolism in a non-respiring eukaryote
Abstract
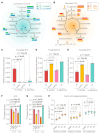
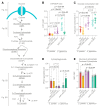
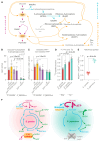
Most eukaryotes respire oxygen, using it to generate biomass and energy. However, a few organisms have lost the capacity to respire. Understanding how they manage biomass and energy production may illuminate the critical points at which respiration feeds into central carbon metabolism and explain possible routes to its optimization. Here, we use two related fission yeasts, Schizosaccharomyces pombe and Schizosaccharomyces japonicus, as a comparative model system. We show that although S. japonicus does not respire oxygen, unlike S. pombe, it is capable of efficient NADH oxidation, amino acid synthesis, and ATP generation. We probe possible optimization strategies through the use of stable isotope tracing metabolomics, mass isotopologue distribution analysis, genetics, and physiological experiments. S. japonicus appears to have optimized cytosolic NADH oxidation via glycerol-3-phosphate synthesis. It runs a fully bifurcated TCA pathway, sustaining amino acid production. Finally, we propose that it has optimized glycolysis to maintain high ATP/ADP ratio, in part by using the pentose phosphate pathway as a glycolytic shunt, reducing allosteric inhibition of glycolysis and supporting biomass generation. By comparing two related organisms with vastly different metabolic strategies, our work highlights the versatility and plasticity of central carbon metabolism in eukaryotes, illuminating critical adaptations supporting the preferential use of glycolysis over oxidative phosphorylation.
Keywords: Schizosaccharomyces japonicus; Schizosaccharomyces pombe; TCA Cycle; bifurcated TCA pathway; fermentation; glycolysis; metabolomics; respiration.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Comment in
-
Metabolism: How a eukaryote adapted to life without respiration.Curr Biol. 2023 Jun 5;33(11):R444-R447. doi: 10.1016/j.cub.2023.05.002. Curr Biol. 2023. PMID: 37279666
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

