Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report
- PMID: 37164270
- PMCID: PMC10735171
- DOI: 10.1016/j.jhep.2023.04.033
Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report
Abstract
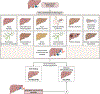
Drug-induced liver injury (DILI) can mimic almost all other liver disorders. A phenotype increasingly ascribed to drugs is autoimmune-like hepatitis (ALH). This article summarises the major topics discussed at a joint International Conference held between the Drug-Induced Liver Injury consortium and the International Autoimmune Hepatitis Group. DI-ALH is a liver injury with laboratory and/or histological features that may be indistinguishable from those of autoimmune hepatitis (AIH). Previous studies have revealed that patients with DI-ALH and those with idiopathic AIH have very similar clinical, biochemical, immunological and histological features. Differentiating DI-ALH from AIH is important as patients with DI-ALH rarely require long-term immunosuppression and the condition often resolves spontaneously after withdrawal of the implicated drug, whereas patients with AIH mostly require long-term immunosuppression. Therefore, revision of the diagnosis on long-term follow-up may be necessary in some cases. More than 40 different drugs including nitrofurantoin, methyldopa, hydralazine, minocycline, infliximab, herbal and dietary supplements (such as Khat and Tinospora cordifolia) have been implicated in DI-ALH. Understanding of DI-ALH is limited by the lack of specific markers of the disease that could allow for a precise diagnosis, while there is similarly no single feature which is diagnostic of AIH. We propose a management algorithm for patients with liver injury and an autoimmune phenotype. There is an urgent need to prospectively evaluate patients with DI-ALH systematically to enable definitive characterisation of this condition.
Keywords: AIH; DI-ALH; DILI; Drug-induced autoimmune-like hepatitis; Liver injury; autoimmune hepatitis; diagnosis; drug-induced liver injury; epidemiology; hepatotoxicity; management; outcome.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have no conflict of interest to disclose in relation with this topic. Arie Regev is employee of Eli Lilly, but has no conflict of interest in relation to this topic.
Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- Andrade RJ, Chalasani N, Björnsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers 2019;5(1):58. - PubMed
-
- European Association for the Study of the Liver. EASL clinical practice guidelines: drug-induced liver injury. J Hepatol 2019;70(6):1222–1261. - PubMed
-
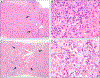
- Lohse AW, Sebode M, Bhathal PS, Clouston AD, Dienes HP, Jain D, et al. Consensus recommendations for histological criteria of autoimmune hepatitis from the international AIH pathology group: results of a workshop on AIH histology hosted by the European reference network on hepatological diseases and the European society of pathology: results of a workshop on AIH histology hosted by the European reference network on hepatological diseases and the European society of pathology. Liver Int 2022;42(5):1058–1069. - PubMed
-
- Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci 2011;56(4):958–976. - PubMed
-
- Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology 2020;72(2):671–722. - PubMed

