Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years
- PMID: 37164640
- PMCID: PMC10441647
- DOI: 10.1124/pharmrev.122.000600
Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years
Erratum in
-
Correction to "Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years".Pharmacol Rev. 2023 Dec 15;76(1):194. doi: 10.1124/pharmrev.122.000600err. Pharmacol Rev. 2023. PMID: 38101936 Free PMC article. No abstract available.
Abstract
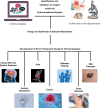
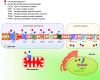
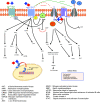
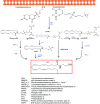
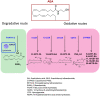
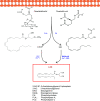
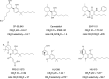
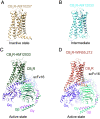
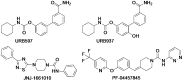
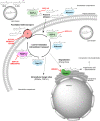
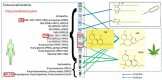
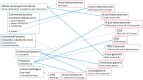
The cannabis derivative marijuana is the most widely used recreational drug in the Western world and is consumed by an estimated 83 million individuals (∼3% of the world population). In recent years, there has been a marked transformation in society regarding the risk perception of cannabis, driven by its legalization and medical use in many states in the United States and worldwide. Compelling research evidence and the Food and Drug Administration cannabis-derived cannabidiol approval for severe childhood epilepsy have confirmed the large therapeutic potential of cannabidiol itself, Δ9-tetrahydrocannabinol and other plant-derived cannabinoids (phytocannabinoids). Of note, our body has a complex endocannabinoid system (ECS)-made of receptors, metabolic enzymes, and transporters-that is also regulated by phytocannabinoids. The first endocannabinoid to be discovered 30 years ago was anandamide (N-arachidonoyl-ethanolamine); since then, distinct elements of the ECS have been the target of drug design programs aimed at curing (or at least slowing down) a number of human diseases, both in the central nervous system and at the periphery. Here a critical review of our knowledge of the goods and bads of the ECS as a therapeutic target is presented to define the benefits of ECS-active phytocannabinoids and ECS-oriented synthetic drugs for human health. SIGNIFICANCE STATEMENT: The endocannabinoid system plays important roles virtually everywhere in our body and is either involved in mediating key processes of central and peripheral diseases or represents a therapeutic target for treatment. Therefore, understanding the structure, function, and pharmacology of the components of this complex system, and in particular of key receptors (like cannabinoid receptors 1 and 2) and metabolic enzymes (like fatty acid amide hydrolase and monoacylglycerol lipase), will advance our understanding of endocannabinoid signaling and activity at molecular, cellular, and system levels, providing new opportunities to treat patients.
Copyright © 2023 by The American Society for Pharmacology and Experimental Therapeutics.
Figures



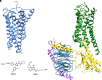







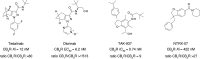





References
-
- Abrams DI, Guzman M (2015) Cannabis in cancer care. Clin Pharmacol ther 97:575–586. - PubMed
-
- Adams R, MacKenzie S Jr, Loewe S (1948) Tetrahydrocannabinol homologs with double branched alkyl groups in the 3-position. J Am Chem Soc 70:664–668. - PubMed
-
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohórquez SM, Stoch A, et al. (2008) The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab 7:68–78. - PubMed
-
- Aghazadeh Tabrizi M, Baraldi PG, Borea PA, Varani K (2016) Medicinal chemistry, pharmacology, and potential therapeutic benefits of cannabinoid CB2 receptor agonists. Chem Rev 116:519–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

