Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration
- PMID: 37165024
- PMCID: PMC10172302
- DOI: 10.1038/s41368-023-00224-5
Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration
Abstract
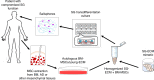
Salivary gland (SG) dysfunction, due to radiotherapy, disease, or aging, is a clinical manifestation that has the potential to cause severe oral and/or systemic diseases and compromise quality of life. Currently, the standard-of-care for this condition remains palliative. A variety of approaches have been employed to restore saliva production, but they have largely failed due to damage to both secretory cells and the extracellular matrix (niche). Transplantation of allogeneic cells from healthy donors has been suggested as a potential solution, but no definitive population of SG stem cells, capable of regenerating the gland, has been identified. Alternatively, mesenchymal stem cells (MSCs) are abundant, well characterized, and during SG development/homeostasis engage in signaling crosstalk with the SG epithelium. Further, the trans-differentiation potential of these cells and their ability to regenerate SG tissues have been demonstrated. However, recent findings suggest that the "immuno-privileged" status of allogeneic adult MSCs may not reflect their status post-transplantation. In contrast, autologous MSCs can be recovered from healthy tissues and do not present a challenge to the recipient's immune system. With recent advances in our ability to expand MSCs in vitro on tissue-specific matrices, autologous MSCs may offer a new therapeutic paradigm for restoration of SG function.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
X-D.C. is a Board member and shareholder in StemBioSys, Inc. (San Antonio, TX). M.M. is a shareholder and member of the Scientific Advisory Board of StemBioSys, Inc. (San Antonio, TX). All other authors have no financial or competing interests to declare.
Figures
Similar articles
-
Organ-specific extracellular matrix directs trans-differentiation of mesenchymal stem cells and formation of salivary gland-like organoids in vivo.Stem Cell Res Ther. 2022 Jul 15;13(1):306. doi: 10.1186/s13287-022-02993-y. Stem Cell Res Ther. 2022. PMID: 35841112 Free PMC article.
-
Injectable decellularized extracellular matrix hydrogel promotes salivary gland regeneration via endogenous stem cell recruitment and suppression of fibrogenesis.Acta Biomater. 2023 Oct 1;169:256-272. doi: 10.1016/j.actbio.2023.08.003. Epub 2023 Aug 7. Acta Biomater. 2023. PMID: 37557943
-
Trends in Salivary Gland Tissue Engineering: From Stem Cells to Secretome and Organoid Bioprinting.Tissue Eng Part B Rev. 2021 Apr;27(2):155-165. doi: 10.1089/ten.TEB.2020.0149. Epub 2020 Aug 26. Tissue Eng Part B Rev. 2021. PMID: 32723016 Review.
-
Mesenchymal Stromal/Stem Cell Therapy Improves Salivary Flow Rate in Radiation-Induced Salivary Gland Hypofunction in Preclinical in vivo Models: A Systematic Review and Meta-Analysis.Stem Cell Rev Rep. 2024 May;20(4):1078-1092. doi: 10.1007/s12015-024-10700-y. Epub 2024 Mar 2. Stem Cell Rev Rep. 2024. PMID: 38430363 Free PMC article.
-
Adipose-derived mesenchymal stem cells regenerate radioiodine-induced salivary gland damage in a murine model.Sci Rep. 2019 Oct 31;9(1):15752. doi: 10.1038/s41598-019-51775-9. Sci Rep. 2019. PMID: 31673085 Free PMC article.
Cited by
-
Salivary gland stem/progenitor cells: advancing from basic science to clinical applications.Cell Regen. 2025 Jan 24;14(1):4. doi: 10.1186/s13619-025-00221-5. Cell Regen. 2025. PMID: 39856475 Free PMC article. Review.
-
Extracellular matrix turnover in salivary gland disorders and regenerative therapies: Obstacles and opportunities.J Oral Biol Craniofac Res. 2023 Nov-Dec;13(6):693-703. doi: 10.1016/j.jobcr.2023.08.009. Epub 2023 Sep 12. J Oral Biol Craniofac Res. 2023. PMID: 37719063 Free PMC article.
-
Development of human salivary gland cell lines for modeling radiation-induced damage in three-dimensional spheroid cultures.J Tissue Eng. 2025 Apr 30;16:20417314251326667. doi: 10.1177/20417314251326667. eCollection 2025 Jan-Dec. J Tissue Eng. 2025. PMID: 40322739 Free PMC article.
-
Research progress in cell therapy for oral diseases: focus on cell sources and strategies to optimize cell function.Front Bioeng Biotechnol. 2024 Mar 7;12:1340728. doi: 10.3389/fbioe.2024.1340728. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38515628 Free PMC article. Review.
-
Immunological subtyping of salivary gland cancer identifies histological origin-specific tumor immune microenvironment.NPJ Precis Oncol. 2024 Jan 20;8(1):15. doi: 10.1038/s41698-024-00501-4. NPJ Precis Oncol. 2024. PMID: 38245623 Free PMC article.
References
-
- Tran, O. N., Wang, H., Dean, D. D., Chen, X.-D. & Yeh, C.-K. Stem cell-based restoration salivary gland function. Chapter 14. In: A Roadmap to Nonhematopoietic Stem Cell-Based Therapeutics. X.-D. Chen (editor), Academic Press, pp. 345–366 (2019). 10.1016/b978-0-12-811920-4.00014-8.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

