Pathogenesis of autoimmune disease
- PMID: 37165096
- PMCID: PMC10171171
- DOI: 10.1038/s41581-023-00720-1
Pathogenesis of autoimmune disease
Abstract
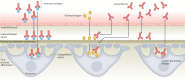
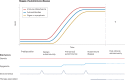
Autoimmune diseases are a diverse group of conditions characterized by aberrant B cell and T cell reactivity to normal constituents of the host. These diseases occur widely and affect individuals of all ages, especially women. Among these diseases, the most prominent immunological manifestation is the production of autoantibodies, which provide valuable biomarkers for diagnosis, classification and disease activity. Although T cells have a key role in pathogenesis, they are technically more difficult to assay. In general, autoimmune disease results from an interplay between a genetic predisposition and environmental factors. Genetic predisposition to autoimmunity is complex and can involve multiple genes that regulate the function of immune cell populations. Less frequently, autoimmunity can result from single-gene mutations that affect key regulatory pathways. Infection seems to be a common trigger for autoimmune disease, although the microbiota can also influence pathogenesis. As shown in seminal studies, patients may express autoantibodies many years before the appearance of clinical or laboratory signs of disease - a period called pre-clinical autoimmunity. Monitoring autoantibody expression in at-risk populations may therefore enable early detection and the initiation of therapy to prevent or attenuate tissue damage. Autoimmunity may not be static, however, and remission can be achieved by some patients treated with current agents.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The author declares no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

