De novo evolution of macroscopic multicellularity
- PMID: 37165189
- PMCID: PMC10425966
- DOI: 10.1038/s41586-023-06052-1
De novo evolution of macroscopic multicellularity
Abstract
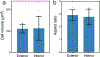
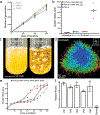
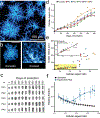
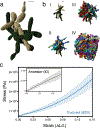
While early multicellular lineages necessarily started out as relatively simple groups of cells, little is known about how they became Darwinian entities capable of sustained multicellular evolution1-3. Here we investigate this with a multicellularity long-term evolution experiment, selecting for larger group size in the snowflake yeast (Saccharomyces cerevisiae) model system. Given the historical importance of oxygen limitation4, our ongoing experiment consists of three metabolic treatments5-anaerobic, obligately aerobic and mixotrophic yeast. After 600 rounds of selection, snowflake yeast in the anaerobic treatment group evolved to be macroscopic, becoming around 2 × 104 times larger (approximately mm scale) and about 104-fold more biophysically tough, while retaining a clonal multicellular life cycle. This occurred through biophysical adaptation-evolution of increasingly elongate cells that initially reduced the strain of cellular packing and then facilitated branch entanglements that enabled groups of cells to stay together even after many cellular bonds fracture. By contrast, snowflake yeast competing for low oxygen5 remained microscopic, evolving to be only around sixfold larger, underscoring the critical role of oxygen levels in the evolution of multicellular size. Together, this research provides unique insights into an ongoing evolutionary transition in individuality, showing how simple groups of cells overcome fundamental biophysical limitations through gradual, yet sustained, multicellular evolution.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures






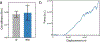



Similar articles
-
Oxygen suppression of macroscopic multicellularity.Nat Commun. 2021 May 14;12(1):2838. doi: 10.1038/s41467-021-23104-0. Nat Commun. 2021. PMID: 33990594 Free PMC article.
-
Evolutionary consequences of nascent multicellular life cycles.Elife. 2023 Oct 27;12:e84336. doi: 10.7554/eLife.84336. Elife. 2023. PMID: 37889142 Free PMC article.
-
Emergence and maintenance of stable coexistence during a long-term multicellular evolution experiment.Nat Ecol Evol. 2024 May;8(5):1010-1020. doi: 10.1038/s41559-024-02367-y. Epub 2024 Mar 14. Nat Ecol Evol. 2024. PMID: 38486107 Free PMC article.
-
Why have aggregative multicellular organisms stayed simple?Curr Genet. 2021 Dec;67(6):871-876. doi: 10.1007/s00294-021-01193-0. Epub 2021 Jun 10. Curr Genet. 2021. PMID: 34114051 Review.
-
Aspects of Multicellularity in Saccharomyces cerevisiae Yeast: A Review of Evolutionary and Physiological Mechanisms.Genes (Basel). 2020 Jun 24;11(6):690. doi: 10.3390/genes11060690. Genes (Basel). 2020. PMID: 32599749 Free PMC article. Review.
Cited by
-
Spontaneous Emergence of Multicellular Heritability.Genes (Basel). 2023 Aug 17;14(8):1635. doi: 10.3390/genes14081635. Genes (Basel). 2023. PMID: 37628687 Free PMC article.
-
Escherichia coli self-organizes developmental rosettes.Proc Natl Acad Sci U S A. 2024 Jun 4;121(23):e2315850121. doi: 10.1073/pnas.2315850121. Epub 2024 May 30. Proc Natl Acad Sci U S A. 2024. PMID: 38814871 Free PMC article.
-
Multicellularity drives ecological diversity in a long-term evolution experiment.Nat Ecol Evol. 2024 May;8(5):856-857. doi: 10.1038/s41559-024-02391-y. Nat Ecol Evol. 2024. PMID: 38519635 No abstract available.
-
Whole-genome duplication in the Multicellularity Long Term Evolution Experiment.bioRxiv [Preprint]. 2024 Apr 19:2024.04.18.588554. doi: 10.1101/2024.04.18.588554. bioRxiv. 2024. Update in: Nature. 2025 Mar;639(8055):691-699. doi: 10.1038/s41586-025-08689-6. PMID: 38659912 Free PMC article. Updated. Preprint.
-
Synthesis of causal and surrogate models by non-equilibrium thermodynamics in biological systems.Sci Rep. 2024 Jan 10;14(1):1001. doi: 10.1038/s41598-024-51426-8. Sci Rep. 2024. PMID: 38200211 Free PMC article.
References
-
- Libby E & Rainey PB. A conceptual framework for the evolutionary origins of multicellularity. Physical biology 10, 035001 (2013). - PubMed
-
- Michod RE in The Evolution of Multicellularity 25–52 (CRC Press, 2022).
-
- Buss LW. The evolution of individuality. Vol. 796 (Princeton University Press, 2014).
-
- Knoll AH. The multiple origins of complex multicellularity. Annual Review of Earth and Planetary Sciences 39, 217–239 (2011).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

