Recombination between heterologous human acrocentric chromosomes
- PMID: 37165241
- PMCID: PMC10172130
- DOI: 10.1038/s41586-023-05976-y
Recombination between heterologous human acrocentric chromosomes
Abstract
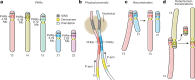
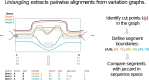
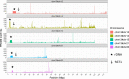
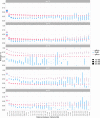
The short arms of the human acrocentric chromosomes 13, 14, 15, 21 and 22 (SAACs) share large homologous regions, including ribosomal DNA repeats and extended segmental duplications1,2. Although the resolution of these regions in the first complete assembly of a human genome-the Telomere-to-Telomere Consortium's CHM13 assembly (T2T-CHM13)-provided a model of their homology3, it remained unclear whether these patterns were ancestral or maintained by ongoing recombination exchange. Here we show that acrocentric chromosomes contain pseudo-homologous regions (PHRs) indicative of recombination between non-homologous sequences. Utilizing an all-to-all comparison of the human pangenome from the Human Pangenome Reference Consortium4 (HPRC), we find that contigs from all of the SAACs form a community. A variation graph5 constructed from centromere-spanning acrocentric contigs indicates the presence of regions in which most contigs appear nearly identical between heterologous acrocentric chromosomes in T2T-CHM13. Except on chromosome 15, we observe faster decay of linkage disequilibrium in the pseudo-homologous regions than in the corresponding short and long arms, indicating higher rates of recombination6,7. The pseudo-homologous regions include sequences that have previously been shown to lie at the breakpoint of Robertsonian translocations8, and their arrangement is compatible with crossover in inverted duplications on chromosomes 13, 14 and 21. The ubiquity of signals of recombination between heterologous acrocentric chromosomes seen in the HPRC draft pangenome suggests that these shared sequences form the basis for recurrent Robertsonian translocations, providing sequence and population-based confirmation of hypotheses first developed from cytogenetic studies 50 years ago9.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











Comment in
-
Human pangenome supports analysis of complex genomic regions.Nature. 2023 May;617(7960):256-258. doi: 10.1038/d41586-023-01490-3. Nature. 2023. PMID: 37165235 No abstract available.

