Porous Materials for Atmospheric Water Harvesting
- PMID: 37165258
- PMCID: PMC10172163
- DOI: 10.1002/open.202300046
Porous Materials for Atmospheric Water Harvesting
Abstract
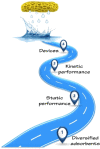
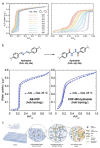
Atmospheric Water Harvesting (AWH) using porous adsorbents is emerging as a promising solution to combat water shortage. Thus, a clearer understanding of the developing trends and optimization strategies of different porous adsorbents can be extremely helpful. Therefore, in this concept, the different types of porous adsorbents and AWH devices are briefly introduced with a focus on the factors that influence the static and kinetic properties of porous adsorbents and their respective optimization strategies. In addition, the fast transport characteristics of water molecules in micropores are studied from the perspective of superfluidity as part of the analysis of the kinetic properties of porous adsorbents. Finally, the future development of porous materials for AWH and the accompanying challenges are summarized.
Keywords: atmospheric water harvesting; kinetic process; porous materials; superfluidity; water adsorption.
© 2023 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- None
-
- Oelkers E. H., Hering J. G., Zhu C., Elements. 2011, 7, 157–162;
-
- Service R. F., Science 2006, 313, 1088–1090. - PubMed
-
- None
Grants and funding
LinkOut - more resources
Full Text Sources

