The involvement of NLRP3 inflammasome in CUMS-induced AD-like pathological changes and related cognitive decline in mice
- PMID: 37165444
- PMCID: PMC10173607
- DOI: 10.1186/s12974-023-02791-0
The involvement of NLRP3 inflammasome in CUMS-induced AD-like pathological changes and related cognitive decline in mice
Abstract
Background: Numerous studies have found that inhibiting the expression of NLRP3 inflammasome can significantly improve depressive-like behaviors in mice, but the research on its effect on cognitive decline in depression and its mechanism is still lacking. This study aimed to elucidate the role of NLRP3 inflammasome in cognitive decline in depression and explore the common neuro-immunological mechanisms of depression and Alzheimer's disease (AD).
Methods: Male C57BL/6 mice were subjected to chronic unpredictable mild stress (CUMS) for 5 weeks, treatment group was administered with the NLRP3 inhibitor MCC950 (10 mg/kg, i.p.), fluoxetine served as positive control. Then, the mice were assessed for cognitive behaviors and depression-like behaviors, and changes of microglia and neurons in hippocampus and levels of Aβ metabolic pathway and tau protein were measured. To explore the mechanism of NLRP3 activation on neurons, we performed in vitro studies using BV2 microglia and mouse primary neurons. Furthermore, we focused on the role of NLRP3 inflammasome in the function of neurons and the expression of AD pathological indicators.
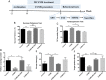
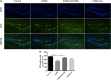
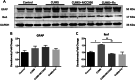
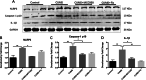
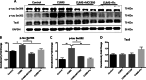
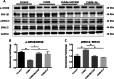
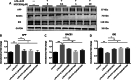
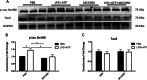
Results: CUMS induced depressive-like behaviors and cognitive decline in mice, which could be reversed by inhibiting NLRP3 inflammasome. MCC950, a specific NLRP3 inhibitor, alleviated CUMS-induced neuron injury and AD-like pathological changes, including the abnormal expression of Aβ metabolic pathway and the hyper-phosphorylation of tau protein. LPS (1 μg/mL) + ATP (1 mM) treatment activated the expression of NLRP3 inflammasome and IL-1β in vitro. In vitro experiment also proved that inhibiting the expression of NLRP3 inflammasome in microglia can restore the Aβ metabolic pathway to normal, decrease neuronal tau protein phosphorylation and protect neurons.
Conclusions: Inhibition of NLRP3 inflammasome effectively alleviated CUMS-induced depressive-like behaviors and cognitive decline in mice, and inhibited the activation of AD physiological indicators.
Keywords: Alzheimer’s disease; Cognitive decline; Depression; NLRP3 inflammasome; Neuroinflammation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

