Alpha-methyltyrosine reduces the acute cardiovascular and behavioral sequelae in a murine model of traumatic brain injury
- PMID: 37165479
- PMCID: PMC10545058
- DOI: 10.1097/TA.0000000000004023
Alpha-methyltyrosine reduces the acute cardiovascular and behavioral sequelae in a murine model of traumatic brain injury
Abstract
Background: Increased catecholamines contribute to heightened cardiovascular reactivity and behavioral deficits after traumatic brain injury (TBI); adrenergic receptor blockade has limited success in reducing adverse sequelae of TBI. Injury-induced increases in the synthesis of catecholamines could contribute to adverse outcomes in TBI. Inhibition of catecholamine synthesis with alpha-methyltyrosine (αMT) could offer a benefit after TBI.
Methods: Original research trial in mice randomized to αMT (50 mg·kg -1 ·d -1 ) or vehicle for 1 week after TBI induced by controlled cortical impact. Primary outcomes of cardiovascular reactivity and behavioral deficits were assessed after 1 week. Secondary outcomes included blood brain barrier permeability and quantification of gene transcription whose products determine intraneuronal chloride concentrations, the release of catecholamines, and activation of the sympathetic nervous system. These genes were the alpha-2 adrenergic receptor ("Adra2c"), the sodium-potassium-chloride cotransporter ("Nkcc1"), and the potassium chloride cotransporter ("Kcc2"). We also assessed the effect of TBI and αMT on the neuronal chloride/bicarbonate exchanger ("Ae3").
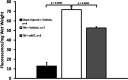
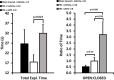
Results: Traumatic brain injury-induced increases in blood pressure and cardiac reactivity were blocked by αMT. Inhibition of catecholamine synthesis decreased blood brain barrier leakage and improved behavioral outcomes after TBI. Traumatic brain injury diminished the transcription of Adra2c and enhanced expression of Nkcc1 while reducing Kcc2 transcription; αMT prevented the induction of the Nkcc1 by TBI without reversing the effects of TBI on Kcc2 expression; αMT also diminished Ae3 transcription.
Conclusion: Traumatic brain injury acutely increases cardiovascular reactivity and induces behavioral deficits in an αMT-sensitive manner, most likely by inducing Nkcc1 gene transcription. Alpha-methyltyrosine may prove salutary in the treatment of TBI by attenuating the enhanced expression of Nkcc1, minimizing blood brain barrier leakage, and diminishing central catecholamine and sympathetic output. We also found an unreported relationship between Kcc2 and the chloride/bicarbonate exchanger, which should be considered in the design of trials planned to manipulate central intraneuronal chloride concentrations following acute brain injury.
Copyright © 2023 Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a “work of the United States Government” for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
Figures




References
-
- Clifton GL, Robertson CS, Kyper K, Taylor AA, Dhekne RD, Grossman RG. Cardiovascular response to severe head injury. J Neurosurg. 1983;59:447–454. - PubMed
-
- Clifton GL, Ziegler MG, Grossman RG. Circulating catecholamines and sympathetic activity after head injury. Neurosurgery. 1981;8:10–14. - PubMed
-
- Kobori N, Clifton GL, Dash PK. Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

