Safety and efficacy of zinpentraxin alfa as monotherapy or in combination with ruxolitinib in myelofibrosis: stage I of a phase II trial
- PMID: 37165840
- PMCID: PMC10543197
- DOI: 10.3324/haematol.2022.282411
Safety and efficacy of zinpentraxin alfa as monotherapy or in combination with ruxolitinib in myelofibrosis: stage I of a phase II trial
Abstract
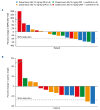
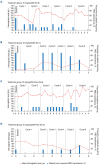
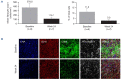
Pentraxin 2 (PTX-2; serum amyloid P component), a circulating endogenous regulator of the inflammatory response to tissue injury and fibrosis, is reduced in patients with myelofibrosis (MF). Zinpentraxin alfa (RO7490677, PRM-151) is a recombinant form of PTX-2 that has shown preclinical antifibrotic activity and no dose-limiting toxicities in phase I trials. We report results from stage 1 of a phase II trial of zinpentraxin alfa in patients with intermediate-1/2 or high-risk MF. Patients (n=27) received intravenous zinpentraxin α weekly (QW) or every 4 weeks (Q4W), as monotherapy or an additional therapy for patients on stable-dose ruxolitinib. The primary endpoint was overall response rate (ORR; investigatorassessed) adapted from International Working Group-Myeloproliferative Neoplasms Research and Treatment criteria. Secondary endpoints included modified Myeloproliferative Neoplasm-Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) change, bone marrow (BM) MF grade reduction, pharmacokinetics, and safety. ORR at week 24 was 33% (n=9/27) and varied across individual cohorts (QW: 38% [3/8]; Q4W: 14% [1/7]; QW+ruxolitinib: 33% [2/6]; Q4W+ruxolitinib: 50% [3/6]). Five of 18 evaluable patients (28%) experienced a ≥50% reduction in MPN-SAF TSS, and six of 17 evaluable patients (35%) had a ≥1 grade improvement from baseline in BM fibrosis at week 24. Most treatment-emergent adverse events (AE) were grade 1-2, most commonly fatigue. Among others, anemia and thrombocytopenia were infrequent (n=3 and n=1, respectively). Treatment-related serious AE occurred in four patients (15%). Overall, zinpentraxin alfa showed evidence of clinical activity and tolerable safety as monotherapy and in combination with ruxolitinib in this open-label, non-randomized trial (clinicaltrials gov. Identifier: NCT01981850).
Figures


References
-
- Moulard O, Mehta J, Fryzek J, Olivares R, Iqbal U, Mesa RA. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol. 2014;92(4):289-297. - PubMed
-
- Cervantes F, Dupriez B, Pereira A, et al. . New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895-2901. - PubMed
-
- Cervantes F. How I treat myelofibrosis. Blood. 2014;124(17):2635-2642. - PubMed
-
- O'Sullivan JM, Harrison CN. Myelofibrosis: clinicopathologic features, prognosis, and management. Clin Adv Hematol Oncol. 2018;16(2):121-131. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

