Functional neuroanatomy of monoaminergic systems in the basolateral nuclear complex of the amygdala: Neuronal targets, receptors, and circuits
- PMID: 37166098
- PMCID: PMC10524224
- DOI: 10.1002/jnr.25201
Functional neuroanatomy of monoaminergic systems in the basolateral nuclear complex of the amygdala: Neuronal targets, receptors, and circuits
Abstract
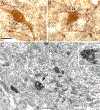
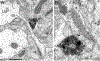
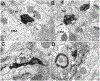
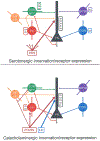
This review discusses neuroanatomical aspects of the three main monoaminergic systems innervating the basolateral nuclear complex (BNC) of the amygdala (serotonergic, noradrenergic, and dopaminergic systems). It mainly focuses on immunohistochemical (IHC) and in situ hybridization (ISH) studies that have analyzed the relationship of specific monoaminergic inputs and their receptors to specific neuronal subtypes in the BNC in order to better understand the anatomical substrates of the monoaminergic modulation of BNC circuitry. First, light and electron microscopic IHC investigations identifying the main BNC neuronal subpopulations and characterizing their local circuitry, including connections with discrete PN compartments and other INs, are reviewed. Then, the relationships of each of the three monoaminergic systems to distinct PN and IN cell types, are examined in detail. For each system, the neuronal targets and their receptor expression are discussed. In addition, pertinent electrophysiological investigations are discussed. The last section of the review compares and contrasts various aspects of each of the three monoaminergic systems. It is concluded that the large number of different receptors, each with a distinct mode of action, expressed by distinct cell types with different connections and functions, should offer innumerable ways to subtlety regulate the activity of the BNC by therapeutic drugs in psychiatric diseases in which there are alterations of BNC monoaminergic modulatory systems, such as in anxiety disorders, depression, and drug addiction. It is suggested that an important area for future studies is to investigate how the three systems interact in concert at the neuronal and neuronal network levels.
Keywords: dopamine; immunohistochemistry; interneurons; norepinephrine; pyramidal neurons; serotonin.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of Interest Statement
The author has no conflict of interest to declare.
Figures














References
-
- Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE (1993) Pharmacological and regional characterization of [3H]LY278584 binding sites in human brain. J Neurochem 60:730–737. - PubMed
-
- Abraham PA, Xing G, Zhang L, Yu EZ, Post R, Gamble EH, Li H (2008) Beta1- and beta2-adrenoceptor induced synaptic facilitation in rat basolateral amygdala. Brain Res 1209:65–73. - PubMed
-
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207. - PubMed
-
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K (2010) Understanding wiring and volume transmission. Brain Res Rev 64:137–159. - PubMed
-
- Amaral DG, Price JL, Pitkanen A, and Carmichael ST (1992) Anatomical organization of the primate amygdala. In: The Amygdala. Neurobiological aspects of emotion, memory, and mental dysfunction. (Aggleton JP, ed), pp. 1–66. New York: Wiley-Liss
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

