Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis
- PMID: 37166104
- PMCID: PMC10208641
- DOI: 10.7554/eLife.85092
Hippo signaling impairs alveolar epithelial regeneration in pulmonary fibrosis
Abstract
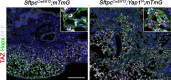
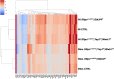
Idiopathic pulmonary fibrosis (IPF) consists of fibrotic alveolar remodeling and progressive loss of pulmonary function. Genetic and experimental evidence indicates that chronic alveolar injury and failure to properly repair the respiratory epithelium are intrinsic to IPF pathogenesis. Loss of alveolar type 2 (AT2) stem cells or mutations that either impair their self-renewal and/or impair their differentiation into AT1 cells can serve as a trigger of pulmonary fibrosis. Recent reports indicate increased YAP activity in respiratory epithelial cells in IPF lungs. Individual IPF epithelial cells with aberrant YAP activation in bronchiolized regions frequently co-express AT1, AT2, conducting airway selective markers and even mesenchymal or EMT markers, demonstrating 'indeterminate' states of differentiation and suggesting that aberrant YAP signaling might promote pulmonary fibrosis. Yet, Yap and Taz have recently also been shown to be important for AT1 cell maintenance and alveolar epithelial regeneration after Streptococcus pneumoniae-induced injury. To investigate how epithelial Yap/Taz might promote pulmonary fibrosis or drive alveolar epithelial regeneration, we inactivated the Hippo pathway in AT2 stem cells resulting in increased nuclear Yap/Taz, and found that this promotes their alveolar regenerative capacity and reduces pulmonary fibrosis following bleomycin injury by pushing them along the AT1 cell lineage. Vice versa, inactivation of both Yap1 and Wwtr1 (encoding Taz) or Wwtr1 alone in AT2 cell stem cells impaired alveolar epithelial regeneration and resulted in increased pulmonary fibrosis upon bleomycin injury. Interestingly, the inactivation of only Yap1 in AT2 stem cells promoted alveolar epithelial regeneration and reduced pulmonary fibrosis. Together, these data suggest that epithelial Yap promotes, and epithelial Taz reduces pulmonary fibrosis suggesting that targeting Yap but not Taz-mediated transcription might help promote AT1 cell regeneration and treat pulmonary fibrosis.
Keywords: Taz; Yap; fibrosis; hippo; lung; mouse; regenerative medicine; stem cell; stem cells.
© 2023, Warren, Lyu et al.
Conflict of interest statement
RW, HL, KK, SD No competing interests declared
Figures

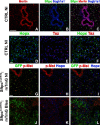
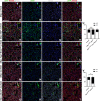
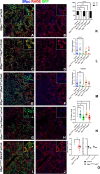
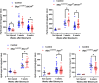






Update of
- doi: 10.1101/2022.11.29.518338
References
-
- Bernau K, Leet JP, Bruhn EM, Tubbs AJ, Zhu T, Sandbo N. Expression of serum response factor in the lung Mesenchyme is essential for development of pulmonary fibrosis. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2021;321:L174–L188. doi: 10.1152/ajplung.00323.2020. - DOI - PMC - PubMed
-
- Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin Alpha6Beta4 identifies an adult distal lung epithelial population with Regenerative potential in mice. The Journal of Clinical Investigation. 2011;121:2855–2862. doi: 10.1172/JCI57673. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

