An Analysis of How Herpes Zoster Pain Affects Health-related Quality of Life of Placebo Patients From 3 Randomized Phase III Studies
- PMID: 37166199
- PMCID: PMC10353534
- DOI: 10.1097/AJP.0000000000001129
An Analysis of How Herpes Zoster Pain Affects Health-related Quality of Life of Placebo Patients From 3 Randomized Phase III Studies
Abstract
Objectives: Herpes zoster (HZ) is a painful condition caused by the reactivation of the varicella-zoster virus, negatively affecting the lives of patients. In this post hoc analysis, we describe the impact of HZ pain on the health-related quality of life (HRQoL) and activities of daily living (ADL) of immunocompetent individuals 50 years of age and older and in hematopoietic stem cell transplantation (HSCT) recipients age 18 years of age and older.
Materials and methods: ZOE-50 (NCT01165177), ZOE-70 (NCT01165229), and ZOE-HSCT (NCT01610414) were phase III, randomized studies conducted in immunocompetent adults 50 years of age and older and 70 years of age and older and in HSCT recipients age 18 years of age and older, respectively. This analysis was performed on patients who experienced an HZ episode in the placebo groups. The impact of varying levels of HZ pain on HRQoL and ADL was analyzed using data from the Zoster Brief Pain Inventory (ZBPI) and the Short Form Health Survey 36 (SF-36) and EQ-5D questionnaires.
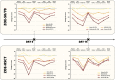
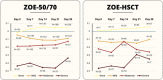
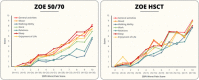
Results: A total of 520 immunocompetent and 172 HSCT individuals with HZ were included. SF-36 and EQ-5D domain scores showed a significant relationship between increased HZ pain and worsening HRQoL. For every increase of 1 in the ZBPI pain score, the estimated mean decrease (worsening) in score in the ZOE-50/70 and ZOE-HSCT, respectively, was 2.0 and 2.4 for SF-36 Role Physical; 2.1 and 1.8 for SF-36 Social Functioning; and 0.041 and 0.045 for EQ-5D utility. Sleep and General activities were the ADL components most affected.
Discussion: Moderate and severe HZ pain had a substantial negative impact on all aspects of HRQoL and ADL. This impact was independent of age and immunosuppression.
Copyright © 2023 GSK. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
GlaxoSmithKline Biologicals SA (Brentford, United Kingdom) funded this study (GSK study identifiers: 110390, 113077) and was involved in all stages of study conduct, including the analysis of the data. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publication of this article. C.B., D.C., E.S.C., and N.L. are employed by/hold shares in GSK. A.L.C. reports a grant from GSK and received honoraria paid to his institution Merck Serono (Merck, Darmstadt, Germany), and BioCSL/Sequirus (Melbourne, Australia) outside the submitted work. S.M. reports personal fees from GSK during the conduct of the study and outside the submitted work. N.L. reports patents planned, issued, or pending outside the submitted work. The remaining authors declare no conflict of interest.
Figures

References
-
- Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57:1–30. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

