Community Synergy of Lactic Acid Bacteria and Cleaner Fermentation of Oat Silage Prepared with a Multispecies Microbial Inoculant
- PMID: 37166312
- PMCID: PMC10269639
- DOI: 10.1128/spectrum.00705-23
Community Synergy of Lactic Acid Bacteria and Cleaner Fermentation of Oat Silage Prepared with a Multispecies Microbial Inoculant
Abstract
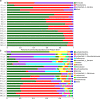
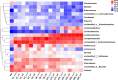
To investigate community synergy of lactic acid bacteria (LAB) and cleaner fermentation of oat silage, oat silages were prepared with or without (control) commercial LAB inoculants LI1 (containing Lactiplantibacillus plantarum, Lentilactobacillus buchneri, Lacticaseibacillus paracasei, and Pediococcus acidilactici) and LI2 (containing Lactiplantibacillus plantarum and Lentilactobacillus buchneri). The microbial community, LAB synergy, and cleaner fermentation were analyzed at 1, 3, 6, 15, 35, and 90 days of ensiling. The LAB inoculant improved fermentation quality, with significantly (P < 0.05) lower pH, ammonia nitrogen content, and gas production and higher lactic acid and acetic acid contents than those of the control. Enterobacteriaceae was the main bacterial community in early stage of fermentation, which utilizes sugar to produce CO2 gas, causing dry matter (DM) and energy loss. As fermentation progressed, the microbial diversity decreased, and the microbial community shifted from Gram-negative to Gram-positive bacteria. The inoculation of multispecies LAB displayed community synergy; Pediococcus acidilactici formed a dominant community in the early stage of fermentation, which produced an acid and anaerobic environment for the subsequent growth of Lentilactobacillus and Lacticaseibacillus species, thus forming a LAB-dominated microbial community. The predicted functional profile indicated that the silage inoculated with LI1 enhanced the carbohydrate metabolism pathway but inhibited the amino acid metabolism pathway, which played a role in promoting faster lactic acid production, reducing the decomposition of protein to ammonia nitrogen, and improving the fermentation quality of silage. Therefore, oat silage can be processed to high-quality and cleaner fermented feed by using an LAB inoculant, and LI1 showed better efficiency than LI2. IMPORTANCE Oat natural silage is rich in Enterobacteriaceae, increasing gas production and fermentation loss. Lactic acid bacteria interact synergistically to form a dominant community during ensiling. Pediococci grow vigorously in the early stage of fermentation and create an anaerobic environment. Lactobacilli inhibit the harmful microorganisms and result in cleaner fermentation of oat silage.
Keywords: clean fermentation; community synergy; gas production; lactic acid bacteria; oat.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Islam MR, Alam AMS, Eneji AE, Ren C, Song W, Hu Y. 2011. Evaluation of a water-saving superabsorbent polymer for forage oat (Avena saliva L.) production in arid regions of northern China. J Food Agric Environ 9:514–518.
-
- Chen L, Guo G, Yuan XJ, Zhang J, Wen AY, Sun XH, Shao T. 2017. Effect of ensiling whole crop oat with Lucerne in different ratios on fermentation quality, aerobic stability and in vitro digestibility on the Tibetan plateau. J Anim Physiol Anim Nutr (Berl) 101:e144–e153. doi: 10.1111/jpn.12577. - DOI - PubMed
-
- Gomes ALM, Jacovaci FA, Bolson DC, Nussio LG, Jobim CC, Daniel JLP. 2019. Effects of light wilting and heterolactic inoculant on the formation of volatile organic compounds, fermentative losses and aerobic stability of oat silage. Anim Feed Sci Technol 247:194–198. doi: 10.1016/j.anifeedsci.2018.11.016. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

