Robust Vaccine-Induced as Well as Hybrid B- and T-Cell Immunity across SARS-CoV-2 Vaccine Platforms in People with HIV
- PMID: 37166335
- PMCID: PMC10269828
- DOI: 10.1128/spectrum.01155-23
Robust Vaccine-Induced as Well as Hybrid B- and T-Cell Immunity across SARS-CoV-2 Vaccine Platforms in People with HIV
Abstract
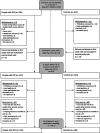
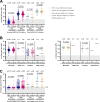
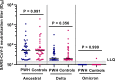
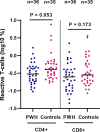
Few studies have comprehensively compared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine-induced and hybrid B- and T-cell responses in people with HIV (PWH) to those in comparable controls without HIV. We included 195 PWH and 246 comparable controls from the AGEhIV COVID-19 substudy. A positive nucleocapsid antibody (INgezim IgA/IgM/IgG) or self-reported PCR test defined prior SARS-CoV-2 infection. SARS-CoV-2 anti-spike (anti-S) IgG titers and anti-S IgG production by memory B cells were assessed. Neutralizing antibody titers were determined in a subset of participants. T-cell responses were assessed by gamma interferon (IFN-γ) release and activation-induced marker assay. We estimated mean differences in postvaccination immune responses (β) between levels of determinants. Anti-S IgG titers and anti-S IgG production by memory B cells were not different between PWH and controls. Prior SARS-CoV-2 infection (β = 0.77), receiving mRNA vaccine (β = 0.56), female sex (β = 0.24), fewer days between last vaccination and sampling (β = 0.07), and a CD4/CD8 ratio of <1.0 (β = -0.39) were independently associated with anti-S IgG titers, but HIV status was not. Neutralization titers against the ancestral and Delta and Omicron SARS-CoV-2 variants were not different between PWH and controls. IFN-γ release was higher in PWH. Prior SARS-CoV-2 infection (β = 2.39), HIV-positive status (β = 1.61), and fewer days between last vaccination and sampling (β = 0.23) were independently associated with higher IFN-γ release. The percentages of SARS-CoV-2-reactive CD4+ and CD8+ T cells, however, were not different between PWH and controls. Individuals with well-controlled HIV generally mount robust vaccine-induced as well as hybrid B- and T-cell immunity across SARS-CoV-2 vaccine platforms similar to controls. Determinants of a reduced vaccine response were likewise largely similar in both groups and included a lower CD4/CD8 ratio. IMPORTANCE Some studies have suggested that people with HIV may respond less well to vaccines against SARS-CoV-2. We comprehensively compared B- and T-cell responses to different COVID-19 vaccines in middle-aged persons with well-treated HIV and individuals of the same age without HIV, who were also highly comparable in terms of demographics and lifestyle, including those with prior SARS-CoV-2 infection. Individuals with HIV generally mounted equally robust immunity to the different vaccines. Even stronger immunity was observed in both groups after prior SARS-CoV-2 infection. These findings are reassuring with respect to the efficacy of SARS-Cov-2 vaccines for the sizable and increasing global population of people with HIV with access and a good response to HIV treatment.
Keywords: HIV; SARS-CoV-2 vaccines; cellular immune responses; humoral immune responses.
Conflict of interest statement
The authors declare a conflict of interest. FWNMW has served on scientific advisory boards for ViiV Healthcare and Gilead sciences. MFSvdL has received independent scientific grant support from Sanofi Pasteur, MSD Janssen Infectious Diseases and Vaccines, and Merck & Co; has served on advisory boards of GlaxoSmithKline and Merck & Co; and has received non-financial support from Stichting Pathologie Onderzoek en Ontwikkeling. MvdV through his institution has received independent scientific grant support and consultancy fees from AbbVie, Gilead Sciences, MSD, and ViiV Healthcare, for which honoraria were all paid to his institution. PR through his institution has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals Inc, Merck & Co and ViiV Healthcare, and has served on scientific advisory boards for Gilead Sciences, ViiV Healthcare, and Merck & Co honoraria for which were all paid to his institution. M.L.V., L.v.P., M.G., A.B., A.C.v.N., K.A.v.D., K.T., J.v.R., M.B., L.v.d.H., M.J.v.G., and N.A.K. declare no competing interests.
Figures

References
-
- Brumme ZL, Mwimanzi F, Lapointe HR, Cheung PK, Sang Y, Duncan MC, Yaseen F, Agafitei O, Ennis S, Ng K, Basra S, Lim LY, Kalikawe R, Speckmaier S, Moran-Garcia N, Young L, Ali H, Ganase B, Umviligihozo G, Omondi FH, Atkinson K, Sudderuddin H, Toy J, Sereda P, Burns L, Costiniuk CT, Cooper C, Anis AH, Leung V, Holmes D, DeMarco ML, Simons J, Hedgcock M, Romney MG, Barrios R, Guillemi S, Brumme CJ, Pantophlet R, Montaner JSG, Niikura M, Harris M, Hull M, Brockman MA. 2022. Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines 7:28. doi: 10.1038/s41541-022-00452-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

