Auxin as an architect of the pectin matrix
- PMID: 37166384
- PMCID: PMC10690733
- DOI: 10.1093/jxb/erad174
Auxin as an architect of the pectin matrix
Abstract
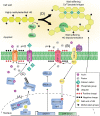
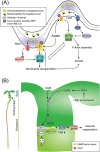
Auxin is a versatile plant growth regulator that triggers multiple signalling pathways at different spatial and temporal resolutions. A plant cell is surrounded by the cell wall, a complex and dynamic network of polysaccharides. The cell wall needs to be rigid to provide mechanical support and protection and highly flexible to allow cell growth and shape acquisition. The modification of the pectin components, among other processes, is a mechanism by which auxin activity alters the mechanical properties of the cell wall. Auxin signalling precisely controls the transcriptional output of several genes encoding pectin remodelling enzymes, their local activity, pectin deposition, and modulation in different developmental contexts. This review examines the mechanism of auxin activity in regulating pectin chemistry at organ, cellular, and subcellular levels across diverse plant species. Moreover, we ask questions that remain to be addressed to fully understand the interplay between auxin and pectin in plant growth and development.
Keywords: Auxin; calcium (Ca2+); cell wall; microdomains; pH; pectin; pectin methylesterase.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

