Obesity alters monocyte developmental trajectories to enhance metastasis
- PMID: 37166450
- PMCID: PMC10182775
- DOI: 10.1084/jem.20220509
Obesity alters monocyte developmental trajectories to enhance metastasis
Abstract
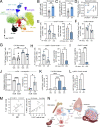
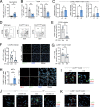
Obesity is characterized by chronic systemic inflammation and enhances cancer metastasis and mortality. Obesity promotes breast cancer metastasis to lung in a neutrophil-dependent manner; however, the upstream regulatory mechanisms of this process remain unknown. Here, we show that obesity-induced monocytes underlie neutrophil activation and breast cancer lung metastasis. Using mass cytometry, obesity favors the expansion of myeloid lineages while restricting lymphoid cells within the peripheral blood. RNA sequencing and flow cytometry revealed that obesity-associated monocytes resemble professional antigen-presenting cells due to a shift in their development and exhibit enhanced MHCII expression and CXCL2 production. Monocyte induction of the CXCL2-CXCR2 axis underlies neutrophil activation and release of neutrophil extracellular traps to promote metastasis, and enhancement of this signaling axis is observed in lung metastases from obese cancer patients. Our findings provide mechanistic insight into the relationship between obesity and cancer by broadening our understanding of the interactive role that myeloid cells play in this process.
© 2023 McDowell et al.
Conflict of interest statement
Disclosures: P.O. Fiset reported consulting fees and honoraria from Amgen, BMS, Merck, AstraZeneca, Pfizer, Astellas, Incyte, EMD Serono, and Hoffmann La Roche outside the submitted work. J.D. Spicer reported consulting fees and honoraria from Roche, Merck, AstraZeneca, BMS, Novartis, Chemocentryx, Amgen, Protalix Biotherapeutics, Xenetic Biosciences, and Regeneron and grants to the institution from Roche, Merck, AstraZeneca, BMS, Protalix Biotherapeutics, and CLS Therapeutics. No other disclosures were reported.
Figures







References
-
- Adrover, J.M., Aroca-Crevillén A., Crainiciuc G., Ostos F., Rojas-Vega Y., Rubio-Ponce A., Cilloniz C., Bonzón-Kulichenko E., Calvo E., Rico D., et al. 2020. Programmed “disarming” of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 21:135–144. 10.1038/s41590-019-0571-2 - DOI - PMC - PubMed
-
- Adrover, J.M., Del Fresno C., Crainiciuc G., Cuartero M.I., Casanova-Acebes M., Weiss L.A., Huerga-Encabo H., Silvestre-Roig C., Rossaint J., Cossío I., et al. 2019. A neutrophil timer coordinates immune defense and vascular protection. Immunity. 50:390–402.e10. 10.1016/j.immuni.2019.01.002 - DOI - PubMed
-
- Alfaro, C., Teijeira A., Oñate C., Pérez G., Sanmamed M.F., Andueza M.P., Alignani D., Labiano S., Azpilikueta A., Rodriguez-Paulete A., et al. 2016. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin. Cancer Res. 22:3924–3936. 10.1158/1078-0432.CCR-15-2463 - DOI - PubMed
-
- Ambrosi, T.H., Scialdone A., Graja A., Gohlke S., Jank A.M., Bocian C., Woelk L., Fan H., Logan D.W., Schürmann A., et al. 2017. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 20:771–784.e6. 10.1016/j.stem.2017.02.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

