PP2A modulation overcomes multidrug resistance in chronic lymphocytic leukemia via mPTP-dependent apoptosis
- PMID: 37166997
- PMCID: PMC10313372
- DOI: 10.1172/JCI155938
PP2A modulation overcomes multidrug resistance in chronic lymphocytic leukemia via mPTP-dependent apoptosis
Abstract
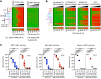
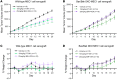
Targeted therapies such as venetoclax (VEN) (Bcl-2 inhibitor) have revolutionized the treatment of chronic lymphocytic leukemia (CLL). We previously reported that persister CLL cells in treated patients overexpress multiple antiapoptotic proteins and display resistance to proapoptotic agents. Here, we demonstrated that multidrug-resistant CLL cells in vivo exhibited apoptosis restriction at a pre-mitochondrial level due to insufficient activation of the Bax and Bak (Bax/Bak) proteins. Co-immunoprecipitation analyses with selective BH domain antagonists revealed that the pleiotropic proapoptotic protein (Bim) was prevented from activating Bax/Bak by "switching" interactions to other upregulated antiapoptotic proteins (Mcl-1, Bcl-xL, Bcl-2). Hence, treatments that bypass Bax/Bak restriction are required to deplete these resistant cells in patients. Protein phosphatase 2A (PP2A) contributes to oncogenesis and treatment resistance. We observed that small-molecule activator of PP2A (SMAP) induced cytotoxicity in multiple cancer cell lines and CLL samples, including multidrug-resistant leukemia and lymphoma cells. The SMAP (DT-061) activated apoptosis in multidrug-resistant CLL cells through induction of mitochondrial permeability transition pores, independent of Bax/Bak. DT-061 inhibited the growth of wild-type and Bax/Bak double-knockout, multidrug-resistant CLL cells in a xenograft mouse model. Collectively, we discovered multidrug-resistant CLL cells in patients and validated a pharmacologically tractable pathway to deplete this reservoir.
Trial registration: ClinicalTrials.gov NCT02419560.
Keywords: Apoptosis pathways; Cancer; Cell Biology; Oncology; Phosphoprotein phosphatases.
Figures




References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

