RWD-derived response in multiple myeloma
- PMID: 37167221
- PMCID: PMC10174483
- DOI: 10.1371/journal.pone.0285125
RWD-derived response in multiple myeloma
Abstract
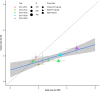
Real-world data (RWD) are important for understanding the treatment course and response patterns of patients with multiple myeloma. This exploratory pilot study establishes a way to reliably assess response from incomplete laboratory measurements captured in RWD. A rule-based algorithm, adapted from International Myeloma Working Group response criteria, was used to derive response using RWD. This derived response (dR) algorithm was assessed using data from the phase III BELLINI trial, comparing the number of responders and non-responders assigned by independent review committee (IRC) versus the dR algorithm. To simulate a real-world scenario with missing data, a sensitivity analysis was conducted whereby available laboratory measurements in the dataset were artificially reduced. Associations between dR and overall survival were evaluated at 1) individual level and 2) treatment level in a real-world patient cohort obtained from a nationwide electronic health record-derived de-identified database. The algorithm's assignment of responders was highly concordant with that of the IRC (Cohen's Kappa 0.83) using the BELLINI data. The dR replicated the differences in overall response rate between the intervention and placebo arms reported in the trial (odds ratio 2.1 vs. 2.3 for IRC vs. dR assessment, respectively). Simulation of missing data in the sensitivity analysis (-50% of available laboratory measurements and -75% of urine monoclonal protein measurements) resulted in a minor reduction in the algorithm's accuracy (Cohen's Kappa 0.75). In the RWD cohort, dR was significantly associated with overall survival at all landmark times (hazard ratios 0.80-0.81, p<0.001) at the individual level, while the overall association was R2 = 0.67 (p<0.001) at the treatment level. This exploratory pilot study demonstrates the feasibility of deriving accurate response from RWD. With further confirmation in independent cohorts, the dR has the potential to be used as an endpoint in real-world studies and as a comparator in single-arm clinical trials.
Copyright: © 2023 Xu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: TX and ST report employment at Roche and Genentech. JR and AS report employment at Flatiron Health, Inc., which is an independent subsidiary of the Roche Group, and report stock ownership in Roche.KM, AR and KY report employment at Roche. MW, WJH and HJ report employment at Genentech. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Palumbo A, Sezer O, Kyle R, Miguel JS, Orlowski RZ, Moreau P, et al. International Myeloma Working Group guidelines for the management of multiple myeloma patients ineligible for standard high-dose chemotherapy with autologous stem cell transplantation. Leukemia. 2009; 23(10): 1716–30. doi: 10.1038/leu.2009.122 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

