Time-dependent vaccine efficacy estimation quantified by a mathematical model
- PMID: 37167285
- PMCID: PMC10174497
- DOI: 10.1371/journal.pone.0285466
Time-dependent vaccine efficacy estimation quantified by a mathematical model
Abstract
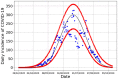
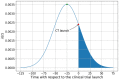
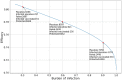
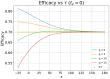
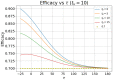
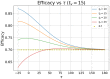
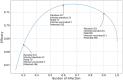
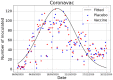
In this paper we calculate the variation of the estimated vaccine efficacy (VE) due to the time-dependent force of infection resulting from the difference between the moment the Clinical Trial (CT) begins and the peak in the outbreak intensity. Using a simple mathematical model we tested the hypothesis that the time difference between the moment the CT begins and the peak in the outbreak intensity determines substantially different values for VE. We exemplify the method with the case of the VE efficacy estimation for one of the vaccines against the new coronavirus SARS-CoV-2.
Copyright: © 2023 Loria et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Scheppler L, De Clercq N, McGoldrick M, Dias J. Regulatory Harmonization and Streamlining of Clinical Trial Applications globally should lead to faster clinical development and earlier access to life-saving vaccines. Vaccine. 2021;. - PubMed
-
- Bellomo N, Bingham R, Chaplain MA, Dosi G, Forni G, Knopoff DA, et al.. A multiscale model of virus pandemic: Heterogeneous interactive entities in a globally connected world. Mathematical Models and Methods in Applied Sciences. 2020;30(08):1591–1651. doi: 10.1142/s0218202520500323 - DOI - PMC - PubMed
-
- Bellomo N, Burini D, Dosi G, Gibelli L, Knopoff D, Outada N, et al.. What is life? A perspective of the mathematical kinetic theory of active particles. Mathematical Models and Methods in Applied Sciences. 2021;31(09):1821–1866. doi: 10.1142/S0218202521500408 - DOI
-
- World Health Organization, Global Immunization Vision and Strategy 2006–2015; 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

