Vaccination-Associated Myocarditis and Myocardial Injury
- PMID: 37167355
- PMCID: PMC10171307
- DOI: 10.1161/CIRCRESAHA.122.321881
Vaccination-Associated Myocarditis and Myocardial Injury
Abstract
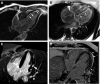
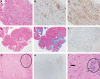
SARS-CoV-2 vaccine-associated myocarditis/myocardial injury should be evaluated in the contexts of COVID-19 infection, other types of viral myocarditis, and other vaccine-associated cardiac disorders. COVID-19 vaccine-associated myocardial injury can be caused by an inflammatory immune cell infiltrate, but other etiologies such as microvascular thrombosis are also possible. The clinical diagnosis is typically based on symptoms and cardiac magnetic resonance imaging. Endomyocardial biopsy is confirmatory for myocarditis, but may not show an inflammatory infiltrate because of rapid resolution or a non-inflammatory etiology. Myocarditis associated with SARS-COVID-19 vaccines occurs primarily with mRNA platform vaccines, which are also the most effective. In persons aged >16 or >12 years the myocarditis estimated crude incidences after the first 2 doses of BNT162b2 and mRNA-1273 are approximately 1.9 and 3.5 per 100 000 individuals, respectively. These rates equate to excess incidences above control populations of approximately 1.2 (BNT162b2) and 1.9 (mRNA-1273) per 100 000 persons, which are lower than the myocarditis rate for smallpox but higher than that for influenza vaccines. In the studies that have included mRNA vaccine and SARS-COVID-19 myocarditis measured by the same methodology, the incidence rate was increased by 3.5-fold over control in COVID-19 compared with 1.5-fold for BNT162b2 and 6.2-fold for mRNA-1273. However, mortality and major morbidity are less and recovery is faster with mRNA vaccine-associated myocarditis compared to COVID-19 infection. The reasons for this include vaccine-associated myocarditis having a higher incidence in young adults and adolescents, typically no involvement of other organs in vaccine-associated myocarditis, and based on comparisons to non-COVID viral myocarditis an inherently more benign clinical course.
Keywords: disorder; infection; inflammatory disease; mRNA vaccine-associated myocarditis.
Conflict of interest statement
Figures



References
-
- Richardson P, McKenna W, Bristow MR, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, et al. . Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841 - PubMed
-
- Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. Circ Res. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

