COVID-19, Myocarditis and Pericarditis
- PMID: 37167363
- PMCID: PMC10171304
- DOI: 10.1161/CIRCRESAHA.123.321878
COVID-19, Myocarditis and Pericarditis
Abstract
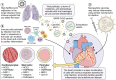
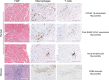
Viral infections are a leading cause of myocarditis and pericarditis worldwide, conditions that frequently coexist. Myocarditis and pericarditis were some of the early comorbidities associated with SARS-CoV-2 infection and COVID-19. Many epidemiologic studies have been conducted since that time concluding that SARS-CoV-2 increased the incidence of myocarditis/pericarditis at least 15× over pre-COVID levels although the condition remains rare. The incidence of myocarditis pre-COVID was reported at 1 to 10 cases/100 000 individuals and with COVID ranging from 150 to 4000 cases/100 000 individuals. Before COVID-19, some vaccines were reported to cause myocarditis and pericarditis in rare cases, but the use of novel mRNA platforms led to a higher number of reported cases than with previous platforms providing new insight into potential pathogenic mechanisms. The incidence of COVID-19 vaccine-associated myocarditis/pericarditis covers a large range depending on the vaccine platform, age, and sex examined. Importantly, the findings highlight that myocarditis occurs predominantly in male patients aged 12 to 40 years regardless of whether the cause was due to a virus-like SARS-CoV-2 or associated with a vaccine-a demographic that has been reported before COVID-19. This review discusses findings from COVID-19 and COVID-19 vaccine-associated myocarditis and pericarditis considering the known symptoms, diagnosis, management, treatment, and pathogenesis of disease that has been gleaned from clinical research and animal models. Sex differences in the immune response to COVID-19 are discussed, and theories for how mRNA vaccines could lead to myocarditis/pericarditis are proposed. Additionally, gaps in our understanding that need further research are raised.
Keywords: COVID-19 vaccines; mRNA vaccines; models, animal; sex characteristics; vaccines.
Conflict of interest statement
Figures


References
-
- Shay DK, Shimabukuro TT, DeStefano F. Myocarditis occurring after immunization with mRNA-based COVID-19 vaccines. JAMA Cardiol. 2021;6:1115–1117. doi: 10.1001/jamacardio.2021.2821 - PubMed
-
- Heidecker B, Dagan N, Balicer R, Eriksson U, Rosano G, Coats A, Tschöpe C, Kelle S, Poland GA, Frustaci A, et al. . Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC working group on myocardial and pericardial diseases. Eur J Heart Fail. 2022;24:2000–2018. doi: 10.1002/ejhf.2669 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

