Mechanisms of skin vascular maturation and maintenance captured by longitudinal imaging of live mice
- PMID: 37167971
- PMCID: PMC10225355
- DOI: 10.1016/j.cell.2023.04.017
Mechanisms of skin vascular maturation and maintenance captured by longitudinal imaging of live mice
Abstract
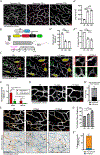
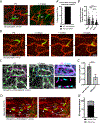
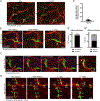
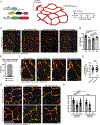
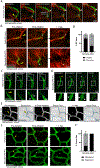
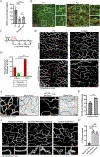
A functional network of blood vessels is essential for organ growth and homeostasis, yet how the vasculature matures and maintains homeostasis remains elusive in live mice. By longitudinally tracking the same neonatal endothelial cells (ECs) over days to weeks, we found that capillary plexus expansion is driven by vessel regression to optimize network perfusion. Neonatal ECs rearrange positions to evenly distribute throughout the developing plexus and become positionally stable in adulthood. Upon local ablation, adult ECs survive through a plasmalemmal self-repair response, while neonatal ECs are predisposed to die. Furthermore, adult ECs reactivate migration to assist vessel repair. Global ablation reveals coordinated maintenance of the adult vascular architecture that allows for eventual network recovery. Lastly, neonatal remodeling and adult maintenance of the skin vascular plexus are orchestrated by temporally restricted, neonatal VEGFR2 signaling. Our work sheds light on fundamental mechanisms that underlie both vascular maturation and adult homeostasis in vivo.
Keywords: angiogenesis; blood flow; capillary; endothelial cell; live imaging; neonatal development; skin; vascular homeostasis; vascular repair; vessel regression.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing financial interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

