Efficacy and safety of three antiretroviral therapy regimens started in pregnancy up to 50 weeks post partum: a multicentre, open-label, randomised, controlled, phase 3 trial
- PMID: 37167996
- PMCID: PMC10280394
- DOI: 10.1016/S2352-3018(23)00061-9
Efficacy and safety of three antiretroviral therapy regimens started in pregnancy up to 50 weeks post partum: a multicentre, open-label, randomised, controlled, phase 3 trial
Abstract
Background: Drugs taken during pregnancy can affect maternal and child health outcomes, but few studies have compared the safety and virological efficacy of different antiretroviral therapy (ART) regimens. We report the primary safety outcomes from enrolment up to 50 weeks post partum and a secondary virological efficacy outcome at 50 weeks post partum of three commonly used ART regimens for HIV-1.
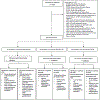
Methods: In this multicentre, open-label, randomised, controlled, phase 3 trial, we enrolled pregnant women aged 18 years or older with confirmed HIV-1 infection at 14-28 weeks of gestation. Women were enrolled at 22 clinical research sites in nine countries (Botswana, Brazil, India, South Africa, Tanzania, Thailand, Uganda, the USA, and Zimbabwe). Participants were randomly assigned (1:1:1) to one of three oral regimens: dolutegravir, emtricitabine, and tenofovir alafenamide; dolutegravir, emtricitabine, and tenofovir disoproxil fumarate; or efavirenz, emtricitabine, and tenofovir disoproxil fumarate. Up to 14 days of antepartum ART before enrolment was permitted. Women with known multiple gestation, fetal anomalies, acute significant illness, transaminases more than 2·5 times the upper limit of normal, or estimated creatinine clearance of less than 60 mL/min were excluded. Primary safety analyses were pairwise comparisons between ART regimens of the proportion of maternal and infant adverse events of grade 3 or higher up to 50 weeks post partum. Secondary efficacy analyses at 50 weeks post partum included a comparison of the proportion of women with plasma HIV-1 RNA of less than 200 copies per mL in the combined dolutegravir-containing groups versus the efavirenz-containing group. Analyses were done in the intention-to-treat population, which included all randomly assigned participants with available data. This trial was registered with ClinicalTrials.gov, NCT03048422.
Findings: Between Jan 19, 2018, and Feb 8, 2019, we randomly assigned 643 pregnant women to the dolutegravir, emtricitabine, and tenofovir alafenamide group (n=217), the dolutegravir, emtricitabine, and tenofovir disoproxil fumarate group (n=215), and the efavirenz, emtricitabine, and tenofovir disoproxil fumarate group (n=211). At enrolment, median gestational age was 21·9 weeks (IQR 18·3-25·3), median CD4 count was 466 cells per μL (308-624), and median HIV-1 RNA was 903 copies per mL (152-5183). 607 (94%) women and 566 (92%) of 617 liveborn infants completed the study. Up to the week 50 post-partum visit, the estimated probability of experiencing an adverse event of grade 3 or higher was 25% in the dolutegravir, emtricitabine, and tenofovir alafenamide group; 31% in the dolutegravir, emtricitabine, and tenofovir disoproxil fumarate group; and 28% in the efavirenz, emtricitabine, and tenofovir disoproxil fumarate group (no significant difference between groups). Among infants, the estimated probability of experiencing at least one adverse event of grade 3 or higher by postnatal week 50 was 28% overall, with small and non-statistically significant differences between groups. By postnatal week 50, 14 infants whose mothers were in the efavirenz-containing group (7%) died, compared with six in the combined dolutegravir groups (1%). 573 (89%) women had HIV-1 RNA data available at 50 weeks post partum: 366 (96%) in the dolutegravir-containing groups and 186 (96%) in the efavirenz-containing group had HIV-1 RNA less than 200 copies per mL, with no significant difference between groups.
Interpretation: Safety and efficacy data during pregnancy and up to 50 weeks post partum support the current recommendation of dolutegravir-based ART (particularly in combination with emtricitabine and tenofovir alafenamide) rather than efavirenz, emtricitabine, and tenofovir disoproxil fumarate, when started in pregnancy.
Funding: National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute of Mental Health.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JvW is an employee of ViiV Healthcare and JFR is an employee of Gilead Sciences. All other authors declare no competing interests.
Figures
Comment in
-
Dolutegravir-based regimens in the post-partum period.Lancet HIV. 2023 Jun;10(6):e352-e353. doi: 10.1016/S2352-3018(23)00086-3. Epub 2023 May 8. Lancet HIV. 2023. PMID: 37167995 No abstract available.
References
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med [Internet]. 1994. Nov 3 [cited 2021 Apr 6];331(18):1173–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7935654 - PubMed
-
- Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. (Accessed Dec 13, 2022).
-
- World Health Organization “Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretrovi. World Health Organization, “Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines: Supplement to the 2016 Consolidated Guideline. 2018.
-
- Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385: 2606–15. - PubMed
-
- Ripin D, Prabhu VR. A cost-savings analysis of a candidate universal antiretroviral regimen. Curr Opin HIV AIDS 2017; 12: 403–07. / - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069456/AI/NIAID NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069530/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- HHSN275201800001I/HD/NICHD NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- T32 AI007509/AI/NIAID NIH HHS/United States
- K24 AI131928/AI/NIAID NIH HHS/United States
- HHSN275201800001C/HD/NICHD NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials