Mesenchymal stromal cell therapy for feline chronic gingivostomatitis: Long term experience
- PMID: 37168097
- PMCID: PMC10165997
- DOI: 10.3389/fvets.2023.1171922
Mesenchymal stromal cell therapy for feline chronic gingivostomatitis: Long term experience
Abstract
Introduction: Mesenchymal stromal cells (MSC) therapy has emerged as a potential treatment option for refractory FCGS. However, there is a lack of long-term data on the use of MSC therapy in cats. This study aimed to evaluate the long-term safety and efficacy of MSC therapy for FCGS and investigate potential factors associated with treatment outcomes.
Methods: This study was a retrospective evaluation of 38 client-owned cats with refractory FCGS who received MSC therapy. Medical records, histopathology, and the Stomatitis Activity Disease Index (SDAI) were reviewed. Correlations of the long-term follow-up success rates with SDAI and cell line type used were conducted. A client survey was also performed to assess side effect occurrence, quality-of-life following treatment, and overall treatment satisfaction.
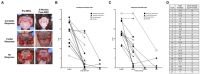
Results: Long-term follow-up ranged from 2 to 9 years post-MSC treatment. The overall positive response rate to MSC treatment was 65.5%, with 58.6% of cats exhibiting permanent improvement or cure. Adverse effects occurring during or immediately after treatment were noted in 34.2% of cases, the majority being transient, self-resolving transfusion-like reactions. No long-term adverse events were noted. No significant correlation in outcome was detected between allogeneic and autologous MSC treatment (p = 0.871) or the severity of the SDAI at entry (p = 0.848) or exit (p = 0.166), or the delta SDAI between entry and exit (p = 0.178). The status 6 months (none to partial improvement vs. substantial improvement to resolution) post-therapy was a predictor of long-term response (value of p < 0.041). Most clients were satisfied with the treatment and outcomes, with 90.6% willing to pursue treatment again, given a similar situation.
Discussion: The results of this study support the use of both autologous and allogeneic MSC as an efficacious and safe therapeutic option for refractory FCGS.
Keywords: MSC; cats; dentistry; gingivostomatitis; regenerative medicine; stromal cells.
Copyright © 2023 Soltero-Rivera, Hart, Blandino, Vapniarsky and Arzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Verhaert L., Van Wetter C. Survey of oral diseases in cats in Flanders-Google Scholar [Internet]. (2004). Available at: https://scholar.google.com/scholar_lookup?title=Survey+of+oral+diseases+...
LinkOut - more resources
Full Text Sources
Miscellaneous

