Temperature dependence of nitrification in a membrane-aerated biofilm reactor
- PMID: 37168114
- PMCID: PMC10165249
- DOI: 10.3389/fmicb.2023.1114647
Temperature dependence of nitrification in a membrane-aerated biofilm reactor
Abstract
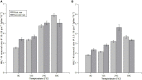
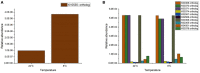
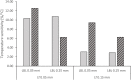
The membrane-aerated biofilm reactor (MABR) is a novel method for the biological treatment of wastewaters and has been successfully applied for nitrification. To improve the design and adaptation of MABR processes for colder climates and varying temperatures, the temperature dependence of a counter-diffusional biofilm's nitrification performance was investigated. A lab-scale MABR system with silicone hollow fibre membranes was operated at various temperatures between 8 and 30°C, and batch tests were performed to determine the ammonia oxidation kinetics. Biofilm samples were taken at 8 and 24°C and analysed with 16S rRNA sequencing to monitor changes in the microbial community composition, and a mathematical model was used to study the temperature dependence of mass transfer. A high nitrification rate (3.08 g N m-2 d-1) was achieved at 8°C, and temperature dependence was found to be low (θ = 1.024-1.026) compared to suspended growth processes. Changes in the community composition were moderate, Nitrospira defluvii remaining the most dominant species. Mass transfer limitations were shown to be largely responsible for the observed trends, consistent with other biofilm processes. The results show that the MABR is a promising technology for low temperature nitrification, and appropriate management of the mass transfer resistance can optimise the process for both low and high temperature operation.
Keywords: community composition; mass transfer; membrane-aerated biofilm; nitrification rate; temperature dependence; wastewater treatment.
Copyright © 2023 Németh, Ainsworth, Ravishankar, Lens and Heffernan.
Conflict of interest statement
AN, JA, and BH were employed by OxyMem Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
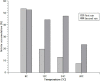


 ) and 0.1–1.0 mg/L (
) and 0.1–1.0 mg/L ( ) NH4+-N concentration at various combinations of biofilm thickness (Lf) and boundary layer thickness (LBL).
) NH4+-N concentration at various combinations of biofilm thickness (Lf) and boundary layer thickness (LBL).
Similar articles
-
Increasing urine nitrification performance with sequential membrane aerated biofilm reactors.Water Res. 2024 Sep 1;261:122019. doi: 10.1016/j.watres.2024.122019. Epub 2024 Jun 29. Water Res. 2024. PMID: 38991244
-
Effect of membrane type on the behavior of nitrifying membrane aerated biofilms: silicone membranes vs. micromembrane cords.Environ Technol. 2024 Mar;45(7):1358-1373. doi: 10.1080/09593330.2022.2143288. Epub 2022 Nov 11. Environ Technol. 2024. PMID: 36318863
-
Performance of pilot-scale membrane aerated biofilm reactors integrated with anoxic nano-biotechnological reactor for domestic wastewater treatment.Chemosphere. 2023 Apr;319:137927. doi: 10.1016/j.chemosphere.2023.137927. Epub 2023 Jan 27. Chemosphere. 2023. PMID: 36716932
-
Recent progress using membrane aerated biofilm reactors for wastewater treatment.Water Sci Technol. 2021 Nov;84(9):2131-2157. doi: 10.2166/wst.2021.443. Water Sci Technol. 2021. PMID: 34810302 Review.
-
The membrane-biofilm reactor (MBfR) as a counter-diffusional biofilm process.Curr Opin Biotechnol. 2016 Apr;38:131-6. doi: 10.1016/j.copbio.2016.01.015. Epub 2016 Feb 12. Curr Opin Biotechnol. 2016. PMID: 26874609 Review.
Cited by
-
Development of a nitrifying bacterial community for a low temperature recirculating aquaculture system.World J Microbiol Biotechnol. 2025 Apr 3;41(4):123. doi: 10.1007/s11274-025-04341-7. World J Microbiol Biotechnol. 2025. PMID: 40175779
-
Diverse reactions of aquaculture biofilter biofilms following acute high-dose peracetic acid.Biofilm. 2025 Apr 2;9:100277. doi: 10.1016/j.bioflm.2025.100277. eCollection 2025 Jun. Biofilm. 2025. PMID: 40242570 Free PMC article.
References
-
- Ahmed W., Delatolla R. (2021). Biofilm and microbiome response of attached growth nitrification systems across incremental decreases to low temperatures. J. Water Proc. Engin. 39:101730. 10.1016/j.jwpe.2020.101730 - DOI
-
- Antoniou P., Hamilton J., Koopman B., Jain R., Holloway B., Lyberatos G., et al. (1990). Effect of temperature and ph on the effective maximum specific growth rate of nitrifying bacteria. Water Res. 24 97–101. 10.1016/0043-1354(90)90070-M - DOI
-
- Ashkanani A., Almomani F., Khraisheh M., Bhosale R., Tawalbeh M., AlJaml K. (2019). Bio-carrier and operating temperature effect on ammonia removal from secondary wastewater effluents using moving bed biofilm reactor (MBBR). Sci. Tot. Environ. 693:133425. 10.1016/j.scitotenv.2019.07.231 - DOI - PubMed
-
- Brindle K., Stephenson T. (1996). Nitrification in a bubbleless oxygen mass transfer membrane bioreactor. Water Sci. Technol. 34 261–267. 10.1016/S0273-1223(96)00813-X - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

