Spotted fever group rickettsiae and Anaplasma phagocytophilum in Borrelia burgdorferi sensu lato seropositive individuals with or without Lyme disease: A retrospective analysis
- PMID: 37168237
- PMCID: PMC10165448
- DOI: 10.1016/j.nmni.2023.101139
Spotted fever group rickettsiae and Anaplasma phagocytophilum in Borrelia burgdorferi sensu lato seropositive individuals with or without Lyme disease: A retrospective analysis
Abstract
Background: The Ixodes ricinus tick is the main vector of Borrelia burgdorferi and tick-borne encephalitis virus in Switzerland. Spotted fever group Rickettsiae (SFG) and Anaplasma phagocytophilum have been detected in Swiss ticks, however, information about the extent and clinical presentation of these infections in humans is scant.
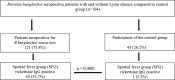
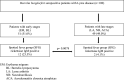
Methods: Indirect fluorescent antibody tests for SFG rickettsiae and Anaplasma phagocytophilum were performed on serum samples of 121 Borrelia burgdorferi seropositive patients with and without Lyme disease and 43 negative controls.
Results: Out of 121 Borrelia burgdorferi seropositive individuals, 65 (53.7%) were seropositive for IgG and 15 (12.4%) for IgM antibodies to SFG rickettsiae. IgM antibodies were detected more frequently in early-than in late-stage of Lyme disease (12 out of 51 and 2 out of 49; respectively; p = 0.0078). Significantly higher IgG antibody titers against SFG rickettsiae were found in patients with late-stage compared to patients with early-stage Lyme disease (mean titer 1:261 and 1:129, respectively; p = 0.038). This difference was even more pronounced in patients with acrodermatitis chronica atrophicans compared to patients with early stage of Lyme disease (mean titer 1:337 and 1:129, respectively; p = 0.009).In patients presenting with fatigue, headache and myalgia, the prevalence of IgG antibodies against SFG rickettsiae was significantly higher (7 out of 11; 63.6%) than in Borrelia burgdorferi seropositive individuals without clinical illness (1 out of 10; 10%; p = 0.024). IgG antibodies to Anaplasma phagocytophilum were detected in 12 out of 121 individuals (9.9%), no IgM antibodies were found.
Conclusion: Infections with SFG rickettsiae and Anaplasma phagocytophilum are underdiagnosed and should be ruled out after a tick bite. Further studies are needed to elucidate the possible causative role of SFG rickettsiae for myalgia, headache and long-lasting fatigue after a tick bite and to determine the necessity for an antibiotic treatment.
Keywords: Acrodermatitis atrophicans chronica; Anaplasma phagocytophilum; Borrelia; Co-infection; Lyme disease; SFG rickettsiae; Switzerland; Tick bite.
© 2023 The Authors.
Figures


References
-
- Walker A.R., Bowman A., Nuttall P. Ticks: biology, disease and control. Parasites Vectors. 2009;2(1) doi: 10.1186/1756-3305-2-1. - DOI
LinkOut - more resources
Full Text Sources
