Refining the Treatment of Pancreatic Cancer From Big Data to Improved Individual Survival
- PMID: 37168490
- PMCID: PMC10165547
- DOI: 10.1093/function/zqad011
Refining the Treatment of Pancreatic Cancer From Big Data to Improved Individual Survival
Abstract
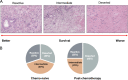
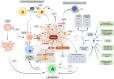
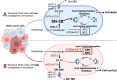
Pancreatic cancer is one of the most lethal cancers worldwide, most notably in Europe and North America. Great strides have been made in combining the most effective conventional therapies to improve survival at least in the short and medium term. The start of treatment can only be made once a diagnosis is made, which at this point, the tumor volume is already very high in the primary cancer and systemically. If caught at the earliest opportunity (in circa 20% patients) surgical resection of the primary followed by combination chemotherapy can achieve 5-year overall survival rates of 30%-50%. A delay in detection of even a few months after symptom onset will result in the tumor having only borderline resectabilty (in 20%-30% of patients), in which case the best survival is achieved by using short-course chemotherapy before tumor resection as well as adjuvant chemotherapy. Once metastases become visible (in 40%-60% of patients), cure is not possible, palliative cytotoxics only being able to prolong life by few months. Even in apparently successful therapy in resected and borderline resectable patients, the recurrence rate is very high. Considerable efforts to understand the nature of pancreatic cancer through large-scale genomics, transcriptomics, and digital profiling, combined with functional preclinical models, using genetically engineered mouse models and patient derived organoids, have identified the critical role of the tumor microenvironment in determining the nature of chemo- and immuno-resistance. This functional understanding has powered fresh and exciting approaches for the treatment of this cancer.
Keywords: CYPY3A; clonal evolution; immunotherapy; molecular subtypes; persister; plasticity; targeted therapies.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
C.S. declares serving on advisory boards for AstraZeneca, Bayer, Eisai, lncyte, MSD, Roche, and Servier. J.P.N. holds the position of Editorial Board Member for Function and is blinded from reviewing or making decisions for the manuscript. The remaining authors have no conflict of interest.
Figures



References
-
- Global Cancer Observatory . International Agency for Research on Cancer. Cancer today. Accessed 12 February 2023. https://gco.iarc.fr/today/home.
-
- Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. 2018;15(6):333–348. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
