Proof-of-concept study for liver-directed miQURE technology in a dyslipidemic mouse model
- PMID: 37168797
- PMCID: PMC10165407
- DOI: 10.1016/j.omtn.2023.04.004
Proof-of-concept study for liver-directed miQURE technology in a dyslipidemic mouse model
Erratum in
-
Erratum: Proof-of-concept study for liver-directed miQURE technology in a dyslipidemic mouse model.Mol Ther Nucleic Acids. 2024 Apr 18;35(2):102189. doi: 10.1016/j.omtn.2024.102189. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38681817 Free PMC article.
Abstract
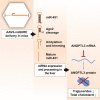
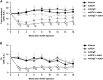
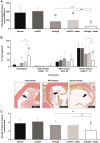
A gene-silencing platform (miQURE) has been developed and successfully used to deliver therapeutic microRNA (miRNA) to the brain, reducing levels of neurodegenerative disease-causing proteins/RNAs via RNA interference and improving the disease phenotype in animal models. This study evaluates the use of miQURE technology to deliver therapeutic miRNA for liver-specific indications. Angiopoietin-like 3 (ANGPTL3) was selected as the target mRNA because it is produced in the liver and because loss-of-function ANGPTL3 mutations and/or pharmacological inhibition of ANGPTL3 protein lowers lipid levels and reduces cardiovascular risk. Overall, 14 candidate miRNA constructs were tested in vitro, the most potent of which (miAngE) was further evaluated in mice. rAAV5-miAngE led to dose-dependent (≤-77%) decreases in Angptl3 mRNA in WT mice with ≤-90% reductions in plasma ANGPTL3 protein. In dyslipidemic APOE∗3-Leiden.CETP mice, AAV5-miAngE significantly reduced cholesterol and triglyceride levels vs. vehicle and scrambled (miSCR) controls when administrated alone, with greater reductions when co-administered with lipid-lowering therapy (atorvastatin). A significant decrease in total atherosclerotic lesion area (-58% vs. miSCR) was observed in AAV5-miAngE-treated dyslipidemic mice, which corresponded with the maintenance of a non-diseased plaque phenotype and reduced lesion severity. These results support the development of this technology for liver-directed indications.
Keywords: AAV; MT: Non-coding RNAs; RNA interference; angiopoietin-like 3 (ANGPTL3); gene therapy; gene-silencing; lipid-lowering; miRNA.
© 2023 The Author(s).
Conflict of interest statement
V.Z. and Y.P.L. are uniQure employees, hold uniQure stocks, and are inventors on the corresponding patent application. A.V., J.M.P.L., L.P., C.V.T., H.W., T.V.Z., K.V.R., M.G., T.G., and E.E. are uniQure employees and hold uniQure stocks. E.J.P., N.K., H.M.G.P., and G.S. are employees of TNO and were employees of TNO during the time this work was conducted.
Figures




References
-
- Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Meister G., Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. - PubMed
-
- Krek A., Grün D., Poy M.N., Wolf R., Rosenberg L., Epstein E.J., MacMenamin P., da Piedade I., Gunsalus K.C., Stoffel M., Rajewsky N. Combinatorial microRNA target predictions. Nat. Genet. 2005;37:495–500. - PubMed
-
- Boudreau R.L., Davidson B.L. Generation of hairpin-based RNAi vectors for biological and therapeutic application. Methods Enzymol. 2012;507:275–296. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

