Metformin mitigates SASP secretion and LPS-triggered hyper-inflammation in Doxorubicin-induced senescent endothelial cells
- PMID: 37168843
- PMCID: PMC10164964
- DOI: 10.3389/fragi.2023.1170434
Metformin mitigates SASP secretion and LPS-triggered hyper-inflammation in Doxorubicin-induced senescent endothelial cells
Abstract
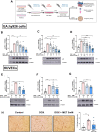
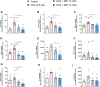
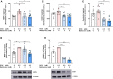
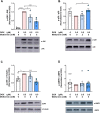
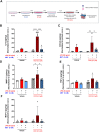
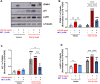
Introduction: Doxorubicin (DOX), a chemotherapeutic drug, induces senescence and increases the secretion of senescence-associated secretory phenotype (SASP) in endothelial cells (ECs), which contributes to DOX-induced inflammaging. Metformin, an anti-diabetic drug, demonstrates senomorphic effects on different models of senescence. However, the effects of metformin on DOX-induced endothelial senescence have not been reported before. Senescent ECs exhibit a hyper-inflammatory response to lipopolysachharide (LPS). Therefore, in our current work, we identified the effects of metformin on DOX-induced endothelial senescence and LPS-induced hyper-inflammation in senescent ECs. Methods: ECs were treated with DOX ± metformin for 24 h followed by 72 h incubation without DOX to establish senescence. Effects of metformin on senescence markers expression, SA-β-gal activity, and SASP secretion were assessed. To delineate the molecular mechanisms, the effects of metformin on major signaling pathways were determined. The effect of LPS ± metformin was determined by stimulating both senescent and non-senescent ECs with LPS for an additional 24 h. Results: Metformin corrected DOX-induced upregulation of senescence markers and decreased the secretion of SASP factors and adhesion molecules. These effects were associated with a significant inhibition of the JNK and NF-κB pathway. A significant hyper-inflammatory response to LPS was observed in DOX-induced senescent ECs compared to non-senescent ECs. Metformin blunted LPS-induced upregulation of pro-inflammatory SASP factors. Conclusion: Our study demonstrates that metformin mitigates DOX-induced endothelial senescence phenotype and ameliorates the hyper-inflammatory response to LPS. These findings suggest that metformin may protect against DOX-induced vascular aging and endothelial dysfunction and ameliorate infection-induced hyper-inflammation in DOX-treated cancer survivors.
Keywords: LPS; SASP; doxorubicin; endothelial senescence; metformin; senomorphics.
Copyright © 2023 Abdelgawad, Agostinucci, Sadaf, Grant and Zordoky.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

