Incidence of Bone Metastases and Skeletal-Related Events in Patients With EGFR-Mutated NSCLC Treated With Osimertinib
- PMID: 37168878
- PMCID: PMC10165134
- DOI: 10.1016/j.jtocrr.2023.100513
Incidence of Bone Metastases and Skeletal-Related Events in Patients With EGFR-Mutated NSCLC Treated With Osimertinib
Abstract
Introduction: Bone metastases are frequent in patients with EGFR-mutated (EGFR+) NSCLC. Skeletal-related events (SREs) are common in these patients; however, no data on SRE in osimertinib-treated patients are reported. We investigated the development of bone metastases and SREs in patients with EGFR+ NSCLC treated with osimertinib.
Methods: This is a retrospective multicenter cohort study that included patients with metastatic EGFR+ NSCLC who were treated with osimertinib between February 2016 and September 2021. Demographics, bone metastases-related outcomes, SREs, treatment efficacy, and overall survival (OS) were collected.
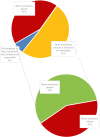
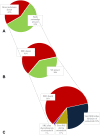
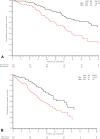
Results: In total, 250 patients treated with osimertinib (43% first line) were included. Of the patients, 51% had bone metastases at initiation of osimertinib. Furthermore, 16% of the patients with bone metastases used bone-targeted agents. Median follow-up from initiation of osimertinib was 23.4 months (95% confidence interval [CI]: 19.9-26.9 mo). During osimertinib treatment, 10% developed new bone metastases or bone progression. Of the patients with bone metastases, 39% had more than or equal to one SREs: 28% developed first SRE before osimertinib treatment, 1% after, and 11% during. Median OS post-bone metastasis was 30.8 months (95% CI: 21.9-39.7). Median OS after first SRE was 31.1 months (95% CI: 15.8-46.5).
Conclusions: Bone metastases and SREs are frequent before and during treatment with osimertinib in EGFR+ NSCLC. Because of these findings and the long OS post-bone metastases, we advocate prescription of bone-targeted agents in these patients and recommend adding bone-specific end points in clinical trials.
Keywords: Bone metastases related outcomes; Bone targeted agents; Lung adenocarcinoma; Solid tumors; Tyrosine kinase inhibitor.
© 2023 The Authors.
Figures
References
-
- Hendriks L.E., Smit E.F., Vosse B.A., et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91. - PubMed
-
- Peters S., Danson S., Hasan B., et al. A randomized open-label phase III trial evaluating the addition of denosumab to standard first-line treatment in advanced NSCLC: the European Thoracic Oncology Platform (ETOP) and European Organisation for Research and Treatment of Cancer (EORTC) SPLENDOUR trial. J Thorac Oncol. 2020;15:1647–1656. - PubMed
-
- Silva G.T., Silva L.M., Bergmann A., Thuler L.C. Bone metastases and skeletal-related events: incidence and prognosis according to histological subtype of lung cancer. Future Oncol. 2019;15:485–494. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

