The TNFΔARE Mouse as a Model of Intestinal Fibrosis
- PMID: 37169343
- PMCID: PMC10433691
- DOI: 10.1016/j.ajpath.2023.04.009
The TNFΔARE Mouse as a Model of Intestinal Fibrosis
Abstract
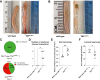
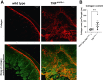
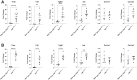
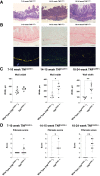
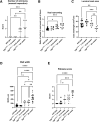
Crohn disease (CD) is a highly morbid chronic inflammatory disease. Although many patients with CD also develop fibrostenosing complications, there are no medical therapies for intestinal fibrosis. This is due, in part, to a lack of high-fidelity biomimetic models to enhance understanding and drug development, which highlights the need for developing in vivo models of inflammatory bowel disease-related intestinal fibrosis. This study investigates whether the TNFΔARE mouse, a model of ileal inflammation, also develops intestinal fibrosis. Several clinically relevant outcomes were studied, including features of structural fibrosis, histologic fibrosis, and gene expression. These include the use of a new luminal casting technique, traditional histologic outcomes, use of second harmonic imaging, and quantitative PCR. These features were studied in aged TNFΔARE mice as well as in cohorts of numerous ages. At >24 weeks of age, TNFΔARE mice developed structural, histologic, and transcriptional changes of ileal fibrosis. Protein and RNA expression profiles showed changes as early as 6 weeks, coinciding with histologic changes as early as 14 to 15 weeks. Overt structural fibrosis was delayed until at least 16 weeks and was most developed after 24 weeks. This study found that the TNFΔARE mouse is a viable and highly tractable model of ileal fibrosis. This model and the techniques used herein can be leveraged for both mechanistic studies and therapeutic development for the treatment of intestinal fibrosis.
Copyright © 2023 American Society for Investigative Pathology. All rights reserved.
Figures


Update of
-
The TNF ΔARE mouse as a model of intestinal fibrosis.bioRxiv [Preprint]. 2023 Jan 14:2023.01.13.523973. doi: 10.1101/2023.01.13.523973. bioRxiv. 2023. Update in: Am J Pathol. 2023 Aug;193(8):1013-1028. doi: 10.1016/j.ajpath.2023.04.009. PMID: 36712048 Free PMC article. Updated. Preprint.
Comment in
-
Re-evaluating the Conclusions of the Study by Steiner et al: Insufficient Evidence to Support TNFΔARE Mice as a Model of Intestinal Fibrosis.Am J Pathol. 2024 Jun;194(6):1154-1155. doi: 10.1016/j.ajpath.2024.03.006. Am J Pathol. 2024. PMID: 38749610 No abstract available.
References
-
- Jairath V., Feagan B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2020;5:2–3. - PubMed
-
- Molodecky N.A., Soon I.S., Rabi D.M., Ghali W.A., Ferris M., Chernoff G., Benchimol E.I., Panaccione R., Ghosh S., Barkema H.W., Kaplan G.G. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. - PubMed
-
- Bettenworth D., Rieder F. Pathogenesis of intestinal fibrosis in inflammatory bowel disease and perspectives for therapeutic implication. Dig Dis. 2017;35:25–31. - PubMed
-
- Strober W., Fuss I.J., Blumberg R.S. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. - PubMed
Publication types
MeSH terms
Grants and funding
- F32 DK122741/DK/NIDDK NIH HHS/United States
- IK6 BX006475/BX/BLRD VA/United States
- UL1 TR002535/TR/NCATS NIH HHS/United States
- R01 DK050189/DK/NIDDK NIH HHS/United States
- IK2 BX005710/BX/BLRD VA/United States
- F32 DK009549/DK/NIDDK NIH HHS/United States
- UL1 TR001082/TR/NCATS NIH HHS/United States
- R01 DK104713/DK/NIDDK NIH HHS/United States
- R01 DK103712/DK/NIDDK NIH HHS/United States
- I01 BX002182/BX/BLRD VA/United States
- R29 DK050189/DK/NIDDK NIH HHS/United States
- R37 DK050189/DK/NIDDK NIH HHS/United States
- R01 DK095491/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

