Angiogenic signaling pathways and anti-angiogenic therapy for cancer
- PMID: 37169756
- PMCID: PMC10175505
- DOI: 10.1038/s41392-023-01460-1
Angiogenic signaling pathways and anti-angiogenic therapy for cancer
Abstract
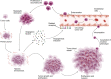
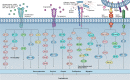
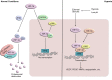
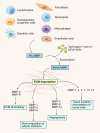
Angiogenesis, the formation of new blood vessels, is a complex and dynamic process regulated by various pro- and anti-angiogenic molecules, which plays a crucial role in tumor growth, invasion, and metastasis. With the advances in molecular and cellular biology, various biomolecules such as growth factors, chemokines, and adhesion factors involved in tumor angiogenesis has gradually been elucidated. Targeted therapeutic research based on these molecules has driven anti-angiogenic treatment to become a promising strategy in anti-tumor therapy. The most widely used anti-angiogenic agents include monoclonal antibodies and tyrosine kinase inhibitors (TKIs) targeting vascular endothelial growth factor (VEGF) pathway. However, the clinical benefit of this modality has still been limited due to several defects such as adverse events, acquired drug resistance, tumor recurrence, and lack of validated biomarkers, which impel further research on mechanisms of tumor angiogenesis, the development of multiple drugs and the combination therapy to figure out how to improve the therapeutic efficacy. Here, we broadly summarize various signaling pathways in tumor angiogenesis and discuss the development and current challenges of anti-angiogenic therapy. We also propose several new promising approaches to improve anti-angiogenic efficacy and provide a perspective for the development and research of anti-angiogenic therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

