Accelerated waning of the humoral response to COVID-19 vaccines in obesity
- PMID: 37169862
- PMCID: PMC10202802
- DOI: 10.1038/s41591-023-02343-2
Accelerated waning of the humoral response to COVID-19 vaccines in obesity
Abstract
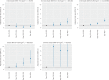
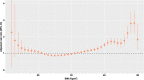
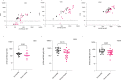
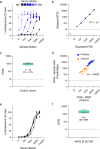
Obesity is associated with an increased risk of severe Coronavirus Disease 2019 (COVID-19) infection and mortality. COVID-19 vaccines reduce the risk of serious COVID-19 outcomes; however, their effectiveness in people with obesity is incompletely understood. We studied the relationship among body mass index (BMI), hospitalization and mortality due to COVID-19 among 3.6 million people in Scotland using the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II) surveillance platform. We found that vaccinated individuals with severe obesity (BMI > 40 kg/m2) were 76% more likely to experience hospitalization or death from COVID-19 (adjusted rate ratio of 1.76 (95% confidence interval (CI), 1.60-1.94). We also conducted a prospective longitudinal study of a cohort of 28 individuals with severe obesity compared to 41 control individuals with normal BMI (BMI 18.5-24.9 kg/m2). We found that 55% of individuals with severe obesity had unquantifiable titers of neutralizing antibody against authentic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus compared to 12% of individuals with normal BMI (P = 0.0003) 6 months after their second vaccine dose. Furthermore, we observed that, for individuals with severe obesity, at any given anti-spike and anti-receptor-binding domain (RBD) antibody level, neutralizing capacity was lower than that of individuals with a normal BMI. Neutralizing capacity was restored by a third dose of vaccine but again declined more rapidly in people with severe obesity. We demonstrate that waning of COVID-19 vaccine-induced humoral immunity is accelerated in individuals with severe obesity. As obesity is associated with increased hospitalization and mortality from breakthrough infections, our findings have implications for vaccine prioritization policies.
© 2023. The Author(s).
Conflict of interest statement
A.S. is a member of the Scottish Government’s Standing Committee on Pandemic Preparedness and the Risk Stratification Subgroup of the UK Government’s New and Emerging Respiratory Virus Threats Advisory Group (NERVTAG). He was a member of AstraZeneca’s Thrombotic Thrombocytopenic Task Force. All roles are unremunerated. S.J.D. is a Scientific Advisor to the Scottish Parliament on COVID-19, for which she receives a fee. I.S.F. has consulted for Eli Lilly, Novo Nordisk and Rhythm Pharmaceuticals on weight loss drugs. All other authors have no conflicts of interest to declare.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/B/000C0433/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/X009297/1/MRC_/Medical Research Council/United Kingdom
- U19 AI082630/AI/NIAID NIH HHS/United States
- MR/W020564/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_0025/12 /MRC_/Medical Research Council/United Kingdom
- 208363/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- MR/T032413/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_19075/MRC_/Medical Research Council/United Kingdom
- MRF-057-0002-RG-THAV-C0798/MRF_/MRF_/United Kingdom
- WT109965MA/WT_/Wellcome Trust/United Kingdom
- MC_PC_20058/MRC_/Medical Research Council/United Kingdom
- MC_PC_19004/MRC_/Medical Research Council/United Kingdom
- BBS/E/B/000C0427/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/000C0428/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 204845/Z/16/Z /WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

