Metabolic influence of core ciliates within the rumen microbiome
- PMID: 37169869
- PMCID: PMC10284877
- DOI: 10.1038/s41396-023-01407-y
Metabolic influence of core ciliates within the rumen microbiome
Abstract
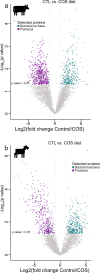
Protozoa comprise a major fraction of the microbial biomass in the rumen microbiome, of which the entodiniomorphs (order: Entodiniomorphida) and holotrichs (order: Vestibuliferida) are consistently observed to be dominant across a diverse genetic and geographical range of ruminant hosts. Despite the apparent core role that protozoal species exert, their major biological and metabolic contributions to rumen function remain largely undescribed in vivo. Here, we have leveraged (meta)genome-centric metaproteomes from rumen fluid samples originating from both cattle and goats fed diets with varying inclusion levels of lipids and starch, to detail the specific metabolic niches that protozoa occupy in the context of their microbial co-habitants. Initial proteome estimations via total protein counts and label-free quantification highlight that entodiniomorph species Entodinium and Epidinium as well as the holotrichs Dasytricha and Isotricha comprise an extensive fraction of the total rumen metaproteome. Proteomic detection of protozoal metabolism such as hydrogenases (Dasytricha, Isotricha, Epidinium, Enoploplastron), carbohydrate-active enzymes (Epidinium, Diplodinium, Enoploplastron, Polyplastron), microbial predation (Entodinium) and volatile fatty acid production (Entodinium and Epidinium) was observed at increased levels in high methane-emitting animals. Despite certain protozoal species having well-established reputations for digesting starch, they were unexpectedly less detectable in low methane emitting-animals fed high starch diets, which were instead dominated by propionate/succinate-producing bacterial populations suspected of being resistant to predation irrespective of host. Finally, we reaffirmed our abovementioned observations in geographically independent datasets, thus illuminating the substantial metabolic influence that under-explored eukaryotic populations have in the rumen, with greater implications for both digestion and methane metabolism.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Martin C, Coppa M, Fougère H, Bougouin A, Baumont R, Eugène M, et al. Diets supplemented with corn oil and wheat starch, marine algae, or hydrogenated palm oil modulate methane emissions similarly in dairy goats and cows, but not feeding behavior. Anim Feed Sci Technol. 2021;272:114783. doi: 10.1016/j.anifeedsci.2020.114783. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

